Nearly 25 % of the world’s population suffers from anaemia, with pregnant women and children of pre-school age the most affected( 1 ). In Uganda, anaemia is the third leading cause of in-patient mortality( 2 ). In 2011, 49 % of under-fives, 23 % of women of child-bearing age and 31 % of pregnant women in Uganda had anaemia( 3 ). Anaemia in developing countries has a multifactorial aetiology which includes Fe deficiency (ID) due to dependence on predominantly plant-based diets from which adequate Fe cannot be absorbed( Reference Gerbens-Leenes, Nonhebel and Krol 4 , Reference Lee, Talegawkar and Merialdi 5 ). Infections such as malaria and hookworm infestation also contribute significantly to the high prevalence of anaemia in many settings( Reference Balarajan, Ramakrishnan and Ozaltin 6 ). Additionally, infection and inflammation defined by raised biomarkers contribute to anaemia as was demonstrated in a study in Malawi in which C-reactive protein (CRP) was elevated in 73·5 % of anaemic pregnant women who were Fe-replete by bone marrow assessment( Reference van den Broek and Letsky 7 ). Hinderaker et al.( Reference Hinderaker, Olsen and Lie 8 ) also found an association between elevated CRP and anaemia in pregnant women in rural Tanzania. More recently, Helicobacter pylori, the most common infection globally, has been associated with the development of anaemia, especially in children( Reference Yuan, Li and Yang 9 , Reference Qu, Huang and Xiong 10 ). Although the available evidence regarding the association between H. pylori infection and anaemia in pregnancy is inconclusive, it highlights the potential public health implications of H. pylori during pregnancy( Reference Farag, Stoltzfus and Khalfan 11 – Reference Malik, Guleria and Kaur 14 ).
Since the discovery of the main Fe regulatory hormone, hepcidin, interest has focused on the response of this hormone to both Fe status and infection( Reference Drakesmith and Prentice 15 ). Briefly, hepcidin acts by binding ferroportin and inducing its degradation, thus inhibiting cellular Fe efflux( Reference Drakesmith and Prentice 15 ). Hepcidin synthesis is reduced in ID, resulting in increased Fe absorption from the diet, while hepcidin expression is upregulated during inflammation, resulting in sequestration of Fe and thus limiting the availability of Fe for erythropoiesis( Reference Ganz and Nemeth 16 ). The recent development of analytical methods for analysis of hepcidin in biological samples has provided an opportunity to include hepcidin as a novel biomarker reflecting Fe status.
In Uganda, pregnant women are exposed to infections such as malaria, helminths, H. pylori and sexually transmitted diseases. The aetiology of anaemia is largely unknown in this population. We therefore set out to describe the aetiology of anaemia in pregnant, HIV-negative Ugandan women. In particular, the aim of the study was to explore factors such as ID and common infections as contributors to anaemia during the first trimester of pregnancy in primi- or secundigravidae using biomarkers of anaemia, ID and infection and/or inflammation.
Participants and methods
Study design and site
The present cross-sectional study was carried out at Kawempe Health Centre (KHC) in Kampala, the capital city of Uganda. Kampala is situated at 00°18′49″N, 32°34′52″E in south-central Uganda on the shores of Lake Victoria and at an altitude of 1200 m. Kawempe is one of five administrative divisions of Kampala District. KHC serves a densely populated, low-income area of Kampala. Malaria in this area is meso-endemic, with two peaks following the rainy seasons in March to May and September to November. The main vector is Anopheles gambiae while the common species is Plasmodium falciparum. According to the Uganda Malaria Indicator Survey of 2009( 17 ), the prevalence of malaria in children under 5 years of age in Kampala is 4·9 % and 7·4 % on the basis of microscopy and a rapid diagnostic test, respectively. The Uganda AIDS Indicator Survey reports that the prevalence of syphilis in pregnant women is 2·4 % and is 1·8 % in women of child-bearing age.( 18 ) The median prevalence of hookworm in the Central Region of Uganda is 6·5 %( Reference Brooker, Kabatereine and Smith 19 ). The prevention and control of anaemia in pregnancy in Uganda is provided for in the National Anemia Policy( 20 ), which specifies the distribution of oral Fe/folic acid supplements, anthelminthics and intermittent presumptive treatment for malaria to pregnant women through antenatal care.
Study participants
Women testing negative for HIV at booking (10–16 weeks’ gestation) at the antenatal care clinic at KHC were invited to participate in the study. Inclusion criteria were: (i) first antenatal visit for the current pregnancy; (ii) Hb >7 g/dl; (iii) gestational age ≤16 weeks; (iv) primi- or secundigravida; and (v) HIV negative. Any woman with pregnancy-related complications or who reported using antibiotics in the 2 weeks preceding the sampling was excluded and referred for care at KHC.
Data collection
After signing the consent form, socio-economic and demographic data were collected through interviews using a questionnaire. Information collected included the woman’s ethnic group, age, parity, reported gestational age, educational level and employment status. Household characteristics such as household size, number of rooms, sources of water for household use, housing materials and ownership of various household assets were also assessed. Body weight was determined to the nearest 100 g using a standard clinical scale and was expressed in kilograms. Gestational age was assessed by the reported last menstrual period and examination of fundal height by experienced midwives at KHC and was expressed in weeks. The study took place from March 2009 to June 2009.
Laboratory tests
HIV testing was carried out as is routinely done for women attending antenatal care at health facilities in Uganda( 21 ). Hb was determined from finger-prick capillary blood using a HemoCue® Haemoglobinometer at the KHC laboratory following the manufacturer’s directions for use. Hb values were corrected for altitude (1190 m above sea level for Kampala City) by subtracting 0·4 g/dl( Reference Nestel 22 ). Results were expressed as Hb (g/dl). A rapid diagnostic test (Syphicheck-WB®; Qualpro Diagnostics, Alto Santacruz, India) was used for syphilis. Results were expressed as syphilis positive or negative. A venous blood sample (~3 ml) was drawn from each participant into Vacutainer tubes without any additives. The samples were transported in a cool box with ice packs to the Biochemistry Department, Makerere University, Kampala (~3 km away from KHC). Serum was separated by centrifugation in the Nutrition Laboratory, Biochemistry Department on the day of sampling, separated into aliquots and frozen at –20°C until analysis within 6 months. Thick smears of venous blood were prepared and stained with Giemsa for the detection of malaria parasites. Parasites were counted against 200 white blood cells by a trained laboratory technologist at KHC. Results were reported as malaria positive (irrespective of parasite density) or negative. Participants were given clean, labelled, plastic bottles with an applicator for collection of stool samples. A portion of the stool sample was examined for hookworm ova within 2 h of collection by an experienced technician at the KHC laboratory using the saline wet mount technique and the formol–diethyl ether sedimentation technique( 23 ). Results were reported as positive or negative for hookworm. In addition, stool samples were transported to the Department of Biochemistry at Makerere University in Kampala on ice packs in a cool box and were analysed within 24 h for H. pylori antigens or were stored frozen (−20°C) until analysis within 7 d. H. pylori infection was determined using the Premier Platinum® Helicobacter pylori stool antigen test (Meridian Bioscience, Nivelles, Belgium), a microplate enzyme-immunoassay for the qualitative detection of H. pylori antigens in stool. The European Helicobacter pylori Study Group has recommended the use of HpSA in the initial diagnosis of H. pylori infection( Reference Malfertheiner, Megraud and O’Morain 24 ). Results were expressed as H. pylori positive or negative.
Serum ferritin concentration was determined using a commercial solid-phase ELISA method (Ramco Laboratories Inc., Stafford, TX, USA). Serum transferrin receptor (sTfR) concentration was determined using a commercial enzyme immunoassay (Ramco Laboratories Inc.). Results were expressed as serum ferritin (μg/l) and serum sTfR (μg/ml), respectively. Controls provided by the manufacturer were run with each plate. Concentrations of CRP and α-1 acid glycoprotein (AGP) were determined by radial immunoassay using the end-point method (Kent Laboratories Inc., Bellingham, WA, USA). The radial immunodiffusion rings were read using a digital reader (The Binding Site Ltd, Birmingham, UK). Results were expressed as serum CRP (mg/l) and serum AGP (g/l), respectively. CRP >5 mg/l and AGP >1 g/l were used as cut-offs to indicate presence of an inflammatory response to infection, according to the manufacturer. A pooled serum sample based on previously collected blood samples was run with each plate to monitor plate-to-plate and day-to-day variation.
Serum samples for hepcidin analysis were stored at −20°C at the Department of Biochemistry, Makerere University from when the first samples were collected (March 2009) until shipped to The Netherlands (April 2010). Recent hepcidin stability studies showed that hepcidin-25 concentrations in serum stored at −20°C did not change significantly for at least 6 months( Reference Laarakkers, Wiegerinck and Klaver 25 ), and seem to remain stable for a longer time (C Laarakkers and D Swinkels, unpublished results). The samples were packed on dry ice and shipped by air to the Department of Laboratory Medicine, Radboud University Medical Center, Nijmegen, The Netherlands. Hepcidin-25 was measured in June and July 2010 by a combination of weak cation exchange chromatography and time-of-flight mass spectrometry (WCX-TOF-MS)( Reference Kroot, Laarakkers and Geurts-Moespot 26 ). An internal standard (synthetic hepcidin-24; Peptide International Inc., Louisville, KY, USA) was used for quantification( Reference Swinkels, Girelli and Laarakkers 27 ). Peptide spectra were generated on a Microflex LT matrix-enhanced laser desorption/ionization (MALDI) TOF-MS platform (Bruker Daltonics, Bremen, Germany). Serum hepcidin-25 concentrations are expressed as nanomoles per litre (nmol/l). The lower limit of detection of this method was 0·5 nmol/l( Reference Kroot, Laarakkers and Geurts-Moespot 26 ).
Ethical considerations
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Makerere University Faculty of Medicine Research and Ethics Committee. Written informed consent was obtained from all participants. Women diagnosed with infections (malaria, hookworm, syphilis, H. pylori) were referred to the health facility for care. All women recruited into the study received Fe/folic acid supplementation following the guidelines of the National Anemia Policy( 20 ). The study supplemented the health facility’s supply of sulfadoxine-pyrimethamine, mebendazole and oral Fe/folic acid supplements.
Data analysis
Principal components analysis was used to assign a weight to the following household assets: type of house, nature of floor and type of roof; ownership of a watch, radio, bicycle, mobile phone, land, domestic animals, a motorized vehicle and television; participant’s employment status; and whether the household hired labourers. The Kaiser–Meyer–Olkin Measure of Sampling Adequacy was 0·69 while Barlett’s Test of Sphericity was statistically significant, indicating significant relationships between the variables. Three components with eigenvalues higher than 1 were extracted; the first accounted for 27·5 % of the total variance and was selected as the socio-economic score. The socio-economic score was ranked and categorized into wealth tertiles (low, middle and high) with the lowest 20 % in the low tertile and about 40 % in the middle and high tertiles( Reference Filmer and Pritchett 28 , Reference Vyas and Kumaranayake 29 ).
Cut-offs for underweight were calculated for each week up to 16 weeks’ gestation according to the approach used by Knabl et al.( Reference Knabl, Riedel and Gmach 30 ) based on the Institute of Medicine/National Research Council guidelines. For normal-weight women, the upper cut-off of adequate gestational weight gain in the first trimester is 3 kg which results in a weekly gain of 0·23 kg in the first 13 weeks of gestation. The upper limit in the remaining 27 weeks of pregnancy is 13 kg which results in 0·48 kg/week from the 14th week onwards. The weight gain for a particular week was then calculated; for example, at week 16 this would be [(13 weeks × 0·23 kg)+(3 weeks×0·48 kg)]=4·4 kg. We then used the cut-off of 45 kg to define pre-pregnancy underweight( 31 ) in order to derive the cut-off for underweight for a particular week. At 16 weeks this was 4·4 kg+45 kg=49·4 kg. A woman whose weight was less than 49·4 kg at 16 weeks of pregnancy was considered underweight.
Anaemia was defined as Hb <11 g/dl( 20 ). ID was defined as ferritin <12 μg/l( 32 ) and ferritin <30 μg/l( Reference Van Den Broek, Letsky and White 33 ). Additionally, ferritin values were adjusted for the effect of the acute-phase response( Reference Thurnham, McCabe and Haldar 34 ). The women were first classified as normal or as having infection/inflammation on the basis of a CRP cut-off of 5 mg/l or an AGP cut-off of 1·0 g/l. A correction factor of 0·668 was obtained as the ratio of the geometric means of the ferritin concentrations of the normal group (n 60) to the infection/inflammation group (n 91). The ferritin values of the infection/inflammation group were then multiplied by the correction factor to give the corrected ferritin values and a cut-off of ferritin <12 μg/l was used to define ID. A cut-off of sTfR >8·3 μg/ml was also used to define ID according to the manufacturer’s specification (Ramco Laboratories Inc.). Fe-deficiency anaemia (IDA) was defined as Hb <11 g/dl with ferritin <12 μg/l, or ferritin <30 μg/l or sTfR >8·3 μg/ml. The ratio of sTfR to log10 ferritin was also calculated. Measurements of serum hepcidin-25 that were below the limit of detection (<0·5 nmol/l) were dealt with using a goodness-of-fit approach in which values for the measurements that are below the limit of detection are selected in such a way that the Shapiro–Wilk W statistic is maximized, i.e. the ‘best-fitting’ normal distribution is produced( Reference Flynn 35 ). Geometric mean values and 95 % confidence intervals were used to describe ferritin and hepcidin concentrations because their distributions were skewed. Spearman’s correlation coefficient was used to measure the correlation between hepcidin, log10 ferritin, Hb, sTfR, CRP and AGP. Pearson’s χ 2 was used to evaluate associations between anaemia and background characteristics and P values<0·05 were considered as statistically significant. Univariate logistic regression was used to assess crude associations between anaemia and the following covariates: socio-economic status, ethnic group, age, H. pylori status, syphilis infection, malaria infection, hookworm infestation, elevated CRP and/or AGP, Fe status and education. The risk factors associated with anaemia following univariate analysis, and H. pylori infection status which was of specific interest, were entered into a multivariable logistic regression to select the final set of independent risk factors for anaemia in pregnancy. We also estimated population-attributable risk (PAR) for these factors using the following formula, where Px is an estimate of the population exposure for case–control studies:
All statistical analyses were performed using the statistical software package SPSS 17·0 for Windows (2008) except for PAR% and associated 95 % confidence intervals, which were estimated using StatsDirect statistical software version 2·8·0 (2013).
Results
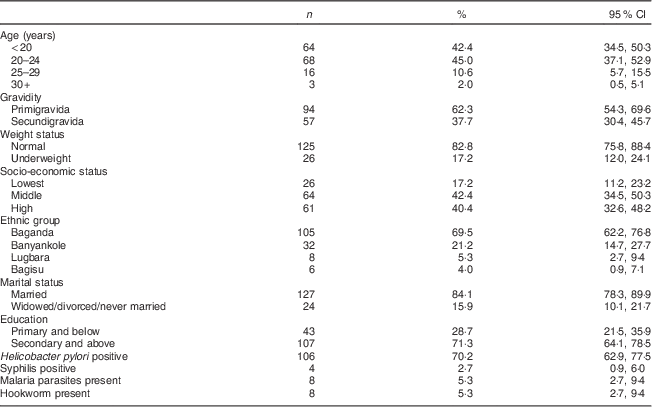
The background characteristics of the 151 mothers enrolled in the study are presented in Table 1. Mean gestational age was 13·7 (sd 2·8) weeks, mean body weight was 57·5 (sd 9·9) kg and 17·2 (95 % CI 12·0, 24·1) % were underweight. Most women were young (over 80 % were under 25 years), two-thirds were primigravidae and a large majority were married. About 70 % had attained secondary education or higher. Almost 70 % of the women were Baganda, which is the main ethnic group in the central region where Kampala City is located. The prevalence of H. pylori infection was 70·2 (95 % CI 62·9, 77·5) %. Four women tested positive for syphilis; eight women tested positive for malaria parasites and for hookworm ova, respectively.
Table 1 Socio-economic and demographic characteristics of the study participants: HIV-negative women (n 151) in their first or second pregnancy at 10–16 weeks’ gestation, attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009
Anaemia and Fe status
Mean Hb was 11·8 (sd 1·7) g/dl and the prevalence of anaemia was 29·1 (95 % CI 21·9, 36·3) %. Geometric mean ferritin concentration was 39·6 (95 % CI 34·1, 46·0) μg/l (Table 2) and 9·9 (95 % CI 5·1, 14·7) % of women had ferritin <12 μg/l. When a higher ferritin cut-off (<30 μg/l) based on previous recommendations for anaemic pregnant African women( Reference Van Den Broek, Letsky and White 33 ) was used, the prevalence of ID was 40·4 (95 % CI 32·9, 48·4) %. The prevalence of ID was 14·6 (95 % CI 9·7, 21·1) % following adjustment of ferritin values for infection/inflammation. Median sTfR was 5·0 (interquartile range (IQR) 4·2–6·4) μg/ml and 6·6 (95 % CI 3·6, 11·0) % of women had serum sTfR >8·3 μg/ml. Median sTfR–ferritin index was 3·14 (IQR 2·5–4·3). Figure 1 shows that 24·5 % of the women had anaemia without ID based on sTfR >8·3 μg/ml while 19·9 % had anaemia without ID based on serum ferritin <30 μg/l and 22·5 % had anaemia without ID based on adjusted serum ferritin <12 μg/l. The prevalence of IDA was 4·6 % based on sTfR >8·3 μg/ml, 9·3 % based on serum ferritin <30 μg/l and 6·6 % based on adjusted serum ferritin <12 μg/l.

Fig. 1 The distribution of anaemia (![]() ), anaemia without iron deficiency (ID;
), anaemia without iron deficiency (ID; ![]() ), ID with anaemia (iron-deficiency anaemia;
), ID with anaemia (iron-deficiency anaemia; ![]() ), ID without anaemia (
), ID without anaemia (![]() ) and ID (with and without anaemia;
) and ID (with and without anaemia; ![]() ) in the study participants on the basis of the indicated biomarkers and cut-offs to define ID. HIV-negative women (n 151) in their first or second pregnancy at 10–16 weeks’ gestation, attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009 (sTfR, serum transferrin receptor)
) in the study participants on the basis of the indicated biomarkers and cut-offs to define ID. HIV-negative women (n 151) in their first or second pregnancy at 10–16 weeks’ gestation, attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009 (sTfR, serum transferrin receptor)
Table 2 Anaemia, iron status and indicators of infection/inflammation among HIV-negative women (n 151) in their first or second pregnancy at 10–16 weeks’ gestation, attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009
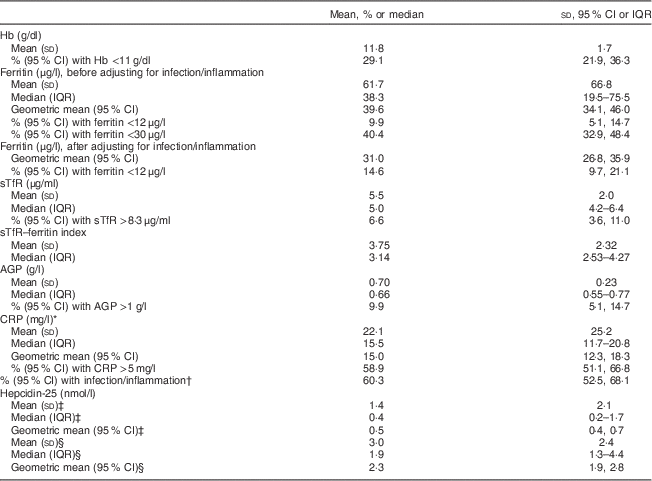
IQR, interquartile range; sTfR, serum transferrin receptor; AGP, α-1 acid glycoprotein; CRP, C-reactive protein.
* Only for those with CRP above the lower effective assay level (n 93).
† AGP and/or CRP greater than 1 g/l and 5 mg/l, respectively.
‡ Values for the measurements of serum hepcidin-25 that were below the limit of detection (<0·5 nmol/l, n 90) were selected using the method described by Flynn( Reference Flynn 35 ).
§ Only for those with hepcidin ≥0·5 nmol/l (n 61).
Anaemia, Fe status and markers of the acute response to infections
Mean AGP was 0·7 (sd 0·23) g/l and 9·9 (95 % CI 5·1, 14·7) % of the women had AGP >1 g/l (Table 2). Mean CRP was markedly raised (22·1 (sd 25·2) mg/l) and 58·9 (95 % CI 51·1, 66·8) % had CRP >5 mg/l. In total, 60·3 (95 % CI 52·5, 68·1) % of the women had elevated AGP and/or CRP concentrations. Figure 2 shows that of the women with anaemia and those without anaemia respectively, 72·7 % and 55·1 % had raised CRP and/or AGP concentrations. The prevalence of raised CRP and/or AGP in the women with non-ID anaemia was 50·0 %, 61·4 % and 56·8 % when ID was defined using ferritin <30 μg/l, sTfR >8·3 μg/ml and adjusted ferritin <12 μg/l, respectively.

Fig. 2 The distribution of iron deficiency (ID) defined with the indicated cut-offs and biomarkers, and infection/inflammation status (![]() ), according to anaemia presence: no ID, normal CRP and AGP (CRP≤5 mg/l and AGP≤ 1 g/l
), according to anaemia presence: no ID, normal CRP and AGP (CRP≤5 mg/l and AGP≤ 1 g/l ![]() ); no ID, elevated CRP and/or AGP (
); no ID, elevated CRP and/or AGP (![]() ); ID, normal CRP and AGP (
); ID, normal CRP and AGP (![]() ); ID, elevated CRP and/or AGP (
); ID, elevated CRP and/or AGP (![]() ). HIV-negative women (n 151; anaemic n 44; non-anaemic n 107) in their first or second pregnancy at 10–16 weeks’ gestation, attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009 (sTfR, serum transferrin receptor; CRP, C-reactive protein; AGP, α-1 acid glycoprotein)
). HIV-negative women (n 151; anaemic n 44; non-anaemic n 107) in their first or second pregnancy at 10–16 weeks’ gestation, attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009 (sTfR, serum transferrin receptor; CRP, C-reactive protein; AGP, α-1 acid glycoprotein)
The concentration of hepcidin in ninety (59·6 %) samples was below the limit of detection (<0·05 nmol/l). After selecting values for measurements of serum hepcidin that were below the limit of detection using the method described by Flynn( Reference Flynn 35 ), geometric mean hepcidin concentration was 0·5 (95 % CI 0·4, 0·7) nmol/l for all samples and 2·3 (95 % CI 1·9, 2·8) nmol/l for the samples that were above the limit of detection (n 61). Hepcidin concentration was positively correlated with ferritin concentration (n 151, r=0·578, P<0·00001; Table 3) and negatively correlated with the sTfR–ferritin index (n 151, r=−0·298, P=0·0001). Ferritin concentration was positively correlated with AGP (n 151, r=0·299, P=0·0001) and negatively correlated with sTfR (n 151, r=−0·162, P=0·02) and sTfR–ferritin index (n 151, r=−0·569, P<0·00001). Hb was negatively correlated with AGP (n 151, r=−0·184, P=0·011) and with the sTfR–ferritin index (n 151, r=−0·249, P=0·001). The difference in mean hepcidin concentration between women with and without ID defined by the different ferritin cut-offs respectively was significant, with a higher mean hepcidin concentration among women with adequate Fe stores (data not shown).
Table 3 Pairwise correlations between Hb, ferritin, sTfR, AGP, CRP and hepcidin among HIV-negative women (n 151) in their first or second pregnancy at 10–16 weeks’ gestation, attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009
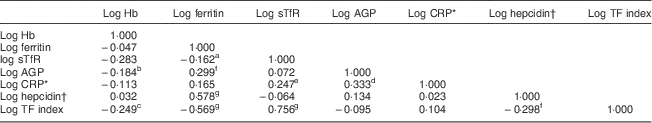
sTfR, serum transferrin receptor; AGP, α-1 acid glycoprotein; CRP, C-reactive protein; TF index, serum transferrin receptor–ferritin index.
* Only for those with CRP above the lower effective assay level (n 93).
† Values for the measurements of serum hepcidin-25 that were below the limit of detection (<0·5 nmol/l, n 90) were selected using the method described by Flynn( Reference Flynn 35 ).
One-tailed significance levels: a P=0·02, b P=0·011, c P=0·001, d P=0·006, e P=0·008, f P=0·0001, g P<0·00001.
Anaemia, malaria, hookworm, H. pylori infection and other factors
Mothers in the 25–29 years age group had slightly lower odds of anaemia (OR=0·12; 95 % CI 0·02, 0·96, P=0·048) relative to mothers in the <20 years age group (Table 4). The odds of anaemia were eight times higher among women with malaria parasites relative to women without malaria parasites (OR=8·29; 95 % CI 1·60, 42·85; P=0·012). Women with raised CRP and/or AGP had higher odds of anaemia relative to women with normal CRP and/or AGP, respectively. Women with ID defined using sTfR >8·3 μg/ml had higher odds of anaemia compared with women without ID (OR=6·56; 95 % CI 1·61, 26·69, P=0·009).
Table 4 Univariate analysis for association between maternal characteristics and anaemia among HIV-negative women (n 151) in their first or second pregnancy at 10–16 weeks’ gestation, attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009
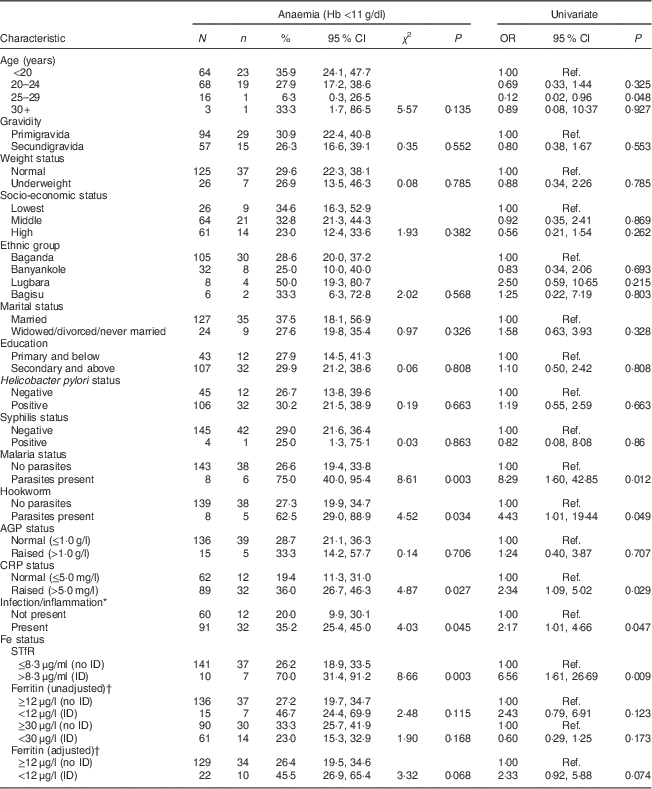
N, number of women in the category, n, number of women in that category who have anaemia; AGP, α-1 acid glycoprotein; CRP, C-reactive protein; sTfR, serum transferrin receptor; ID, iron deficiency; Ref., reference category.
* Infection/inflammation present when AGP and/or CRP is/are raised.
† Adjusted for infection/inflammation.
Multivariate analysis showed that the presence of malaria parasites was independently and positively associated with anaemia irrespective of the biomarker used to define ID (Table 5). ID defined using sTfR >8·3 μg/ml (OR=5·58; 95 % CI 1·26, 24·80) and adjusted ferritin <12 μg/l (OR=2·86; 95 % CI 1·08, 7·52) was independently and positively associated with anaemia, while the presence of hookworm was independently and positively associated with anaemia only when adjusted ferritin was used as a biomarker for ID (OR=6·08; 95 % CI 1·32, 27·93). Table 5 shows that raised CRP had the largest PAR for anaemia (41·6 %; 95 % CI 11·1, 72·2 %) followed by ID defined by sTfR >8·3 μg/ml (13·5 %; 95 % CI 2·0, 25·0 %) and malaria (12·0 %; 95 % CI 1·4, 22·6 %).
Table 5 Multivariate analysis for factors associated with anaemia at 10–16 weeks of pregnancy in HIV-negative women attending the antenatal care clinic at Kawempe Health Centre, Kampala, Uganda, March 2009 to June 2009 (n 151) and their population-attributable risk
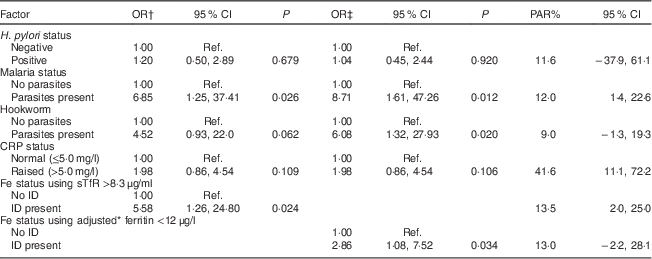
PAR, population-attributable risk; CRP, C-reactive protein; sTfR, serum transferrin receptor; ID, iron deficiency; Ref., reference category.
* Adjusted for infection/inflammation.
† Variables in the analysis: H. pylori status, malaria status, hookworm, CRP status, Fe status using sTfR >8·3 μg/ml.
‡ Variables in the analysis: H. pylori status, malaria status, hookworm, CRP status, Fe status using adjusted ferritin <12 μg/l.
Discussion
As expected, anaemia, infections and/or inflammation were common in this study population. The prevalence of anaemia was 29·1 % which is comparable to 31 % reported in the Uganda Demographic and Health Survey 2011( 3 ) and is consistent with data from other studies in Uganda( Reference Ndyomugyenyi, Kabatereine and Olsen 36 – Reference Ndibazza, Muhangi and Akishule 39 ). However, IDA represented only a small proportion of all anaemia, about 5–10 % depending on the biomarker used and cut-off level applied. Also as expected, ferritin values were widely dispersed and the distribution was skewed to the right. When ID was defined with the commonly used cut-off (<12 μg/l) the prevalence of ID was 9·9 %. When a higher cut-off was used, <30 μg/l, based on Van den Broek et al.’s study of anaemic, pregnant Malawian women using the gold standard bone marrow smear test to define Fe status( Reference Van Den Broek, Letsky and White 33 ), the prevalence of ID was 40·4 %. A previous study in Masindi in western Uganda reported similar findings, as the prevalence of ID among primi- and secundigravidae was 27·3 % based on 30 μg/l as the ferritin cut-off for ID( Reference Ndyomugyenyi, Kabatereine and Olsen 36 ). Our second measure of Fe status was sTfR, which is a specific and sensitive marker of Fe deficiency in pregnancy at the cut-off recommended by the manufacturer( Reference Akesson, Bjellerup and Berglund 40 ). Given that available commercial immunoassays for sTfR are not standardized( Reference Thorpe, Heath and Sharp 41 ), it is only possible to compare sTfR concentrations across studies that have used the same commercial kit. Mean sTfR concentration obtained in the current study compares well with findings from studies that have used the Ramco sTfR kit( Reference Akesson, Bjellerup and Berglund 42 – Reference Schulze, Christian and Ruczinski 45 ). Serum sTfR concentration does not appear to be affected by infectious or inflammatory conditions( Reference Ferguson, Skikne and Simpson 46 ) except for malaria, which in some studies has been observed to increase sTfR levels( Reference Verhoef, West and Ndeto 47 – Reference Righetti, Wegmuller and Glinz 49 ) while in at least one other study caused a decrease in sTfR( Reference Williams, Maitland and Rees 50 ). We did not find any difference in sTfR levels by malaria status or by infection/inflammation (data not shown). ID defined using sTfR and using adjusted ferritin was independently associated with anaemia in pregnancy.
Median hepcidin concentration in our study population was lower than what van Santen et al.( Reference van Santen, Kroot and Zijderveld 51 ) found in the first trimester in a longitudinal study of thirty-one healthy pregnant women in The Netherlands with normal haematological blood counts. Of note, the hepcidin concentrations in their study decreased to undetectable levels in the third trimester, and this was paralleled by decreasing Hb. Given that mean Hb was lower in our study and that nearly a third of the women had anaemia, it is not unexpected that median hepcidin concentration is also lower. Median hepcidin concentrations in both studies were lower than the reference values from non-pregnant, non-anaemic women without infection and/or inflammation, defined as CRP <10 mg/l( Reference Galesloot, Vermeulen and Geurts-Moespot 52 ). A subset of this reference study population, without ID (ferritin >30 μg/l), had an even higher serum hepcidin level. The production of hepcidin is regulated by Fe, with hepcidin synthesis being suppressed during ID, thus allowing more Fe to enter plasma( Reference Ganz and Nemeth 16 ). Hepcidin production is also suppressed during active erythropoiesis, making more Fe available for Hb synthesis( Reference Ganz and Nemeth 16 ). Thus, the substantially lower hepcidin concentration we found reflects active erythropoiesis and the increased demand for Fe during pregnancy. Moreover, mean hepcidin was significantly lower among women with ID compared with women with adequate Fe stores when ID was defined using either ferritin cut-off even though ferritin concentrations were raised apparently due to infection/inflammation and not necessarily because Fe stores were adequate. This seems to suggest that in pregnancy, the need for Fe overrides the normal response of hepcidin to infection/inflammation which causes an increase in hepcidin( Reference Ganz and Nemeth 16 ). Hepcidin had a significant positive correlation with ferritin and a significant negative correlation with the sTfR–ferritin index, and was not correlated with CRP and sTfR in the present study. Similar findings were obtained in pregnant women in Bangladesh( Reference Schulze, Christian and Ruczinski 45 ) and in healthy pregnant women in The Netherlands( Reference van Santen, Kroot and Zijderveld 51 ). In contrast, a study of Turkish pregnant women did not find any relationship between hepcidin and ferritin( Reference Simavli, Uysal and Uysal 53 ). Given that serum ferritin is recognized as the most important correlate of serum hepcidin concentration( Reference Galesloot, Vermeulen and Geurts-Moespot 52 ), the latter results can be ascribed to limitations in the commercial assay used( Reference Kroot, Tjalsma and Fleming 54 ).
Mean CRP concentration was markedly raised and nearly two-thirds of the women had elevated CRP, defined as >5 mg/l. A high prevalence of infection and/or inflammation, indicated by elevated CRP, was also reported among 150 anaemic pregnant women in Malawi as mean CRP concentration was 30 mg/l and 35 % had elevated CRP (CRP >190 nmol/l (19·5 mg/l))( Reference van den Broek and Letsky 7 ). Although CRP increases during normal pregnancy( Reference Teran, Escudero and Calle 55 , Reference Larsson, Palm and Hansson 56 ), these findings in pregnant women in Africa indicate higher concentrations than in other populations. Furthermore, a study in the USA reported that while median CRP was 4·8 mg/l during pregnancy, black race was independently associated with serum CRP levels greater than the 75th percentile (15·7 mg/l)( Reference Picklesimer, Jared and Moss 57 ). A systematic review has shown that race/ethnicity is independently associated with CRP concentrations, and that people of African, Latin or South Asian descent are at higher risk for elevated CRP( Reference Nazmi and Victora 58 ). Thus, the high prevalence of elevated CRP concentration in our pregnant, black African population is potentially partly due to effects of physiological state and race in addition to the influence of infection/inflammation.
Over two-thirds of the women with anaemia also had raised AGP and/or CRP. Over two-thirds of the anaemic women were not Fe-deficient on the basis of either ferritin <30 μg/l, adjusted ferritin <12 μg/l or sTfR >8·3 μg/l. Furthermore, more than half of the anaemic women who did not have ID based on the indicators used in the study had raised AGP and/or CRP. In Malawi, 73·5 % of anaemic pregnant women who were Fe-replete by bone marrow assessment had raised CRP( Reference van den Broek and Letsky 7 ). Another study in Côte d’Ivoire found that IDA accounted for only ~50 % of anaemia in non-pregnant women and adult men( Reference Asobayire, Adou and Davidsson 59 ). We also found that the PAR of CRP for anaemia was 41 %. In other words, 41 % of anaemia in our population would not have occurred if CRP levels were normal (reflecting absence of infection/inflammation). These findings highlight the significant role of infection/inflammation in anaemia in our setting, implying that infection/inflammation should be considered in the strategies currently being used to control and prevent anaemia.
Only eight (5·3 %) women had malaria parasites, a finding which is consistent with the Uganda Malaria Indicator Survey of 2009( 17 ). In spite of the low prevalence, malaria was independently associated with anaemia, albeit with a wide confidence interval. Only four women (2·7 %) tested positive for syphilis at enrolment, which was unexpectedly low for this community. However, the recently released AIDS Indicator Survey reported a low prevalence of syphilis in pregnant women (2·4 %) and in women of child-bearing age (1·8 %) in Uganda.( 18 ) Hookworm infestation was also low at 5 %, which is surprising as overcrowding and limited access to adequate water and sanitation are characteristic of the Kawempe area. Available data for Uganda indicate substantially higher rates of hookworm infestation in different settings( Reference Ndyomugyenyi, Kabatereine and Olsen 36 , Reference Muhangi, Woodburn and Omara 60 – Reference Shapiro, Tukahebwa and Kasten 62 ). The atlas of human helminths in East Africa reports that at 6·5 %, the Central Region has the lowest prevalence of hookworm infestation in Uganda( Reference Brooker, Kabatereine and Smith 19 ). Rural–urban differences could account for the low prevalence we found as lower prevalence of hookworm infestation has been demonstrated in urban areas compared with rural areas( Reference Ayoya, Spiekermann-Brouwer and Traore 63 – Reference Oninla, Owa and Onayade 65 ), although contradictory data have been reported from Zanzibar( Reference Knopp, Mohammed and Stothard 66 ). It is also possible that the prevalence of hookworm ova was underestimated in our study because only one stool sample was examined per participant. If the intensity of infection is low this may lead to false negative results due to sparse and unequal distribution of hookworm ova in stools and variation in the excretion of ova across days( Reference Knopp, Mgeni and Khamis 67 , Reference Goodman, Haji and Bickle 68 ). This may account for the absence of an independent association between hookworm infestation and anaemia in our study.
The prevalence of H. pylori infection, based on the stool antigen test, was 70·2 %. A survey to provide background information to the current study found a prevalence of 45·4 % in pregnant women attending antenatal care in five districts in Uganda and 60·5 % among women enrolled at KHC (RK Baingana, unpublished results). Other data on the prevalence of H. pylori infection in Uganda are from children aged 12 years and below( Reference Gupta, Perez-Perez and Dorsey 69 , Reference Hestvik, Tylleskar and Kaddu-Mulindwa 70 ) and from patients referred for endoscopy( Reference Ochama 71 ) or who had cancer and benign tumours( Reference Newton, Ziegler and Casabonne 72 ), and are not representative of the general population. Our findings are consistent with data from Africa and other developing country settings( Reference Goh, Chan and Shiota 73 ).
On the basis of case studies, observational studies and interventional trials in children and non-pregnant adolescents and adults, it has been suggested that H. pylori infection has a role in the aetiology of anaemia. A retrospective study in Germany found that after adjusting for multiple covariates, mean Hb at the beginning of pregnancy was lower among H. pylori-infected women than among uninfected women( Reference Weyermann, Rothenbacher and Gayer 13 ). Anaemic, pregnant women in Turkey were H. pylori-positive( Reference Mulayim, Celik and Yanik 12 ) while pregnant women with severe anaemia in Zanzibar had nearly eightfold higher odds of H. pylori infection compared with women without severe anaemia( Reference Farag, Stoltzfus and Khalfan 11 ). A randomized controlled pilot trial of H. pylori therapy in pregnant women with IDA in India showed that H. pylori eradication resulted in a significantly better response to Fe supplementation( Reference Malik, Guleria and Kaur 14 ). However, we did not find evidence of an association between H. pylori infection and anaemia. The relationship between H. pylori infection and anaemia may be influenced by the degree of anaemia and by the relative contribution ID makes to anaemia in the population under study( Reference Figura, Franceschi and Santucci 74 ). This probably accounts for our finding because women with severe anaemia were not included in our study and IDA comprised only a small proportion of anaemia in our study population.
A limitation of our study is that we did not explore other dietary factors associated with anaemia such as vitamin A, vitamin B12 and folate deficiency. The prevalence of vitamin A deficiency among women in Kampala in 2006 was 14 %( 75 ) and 47 % of women had inadequate usual intakes of vitamin A( Reference Harvey, Rambeloson and Dary 76 ). Data on the prevalence of folate and vitamin B12 deficiencies are scanty; however, 7 % and 64 % of women in Kampala had inadequate usual intakes( Reference Harvey, Rambeloson and Dary 76 ) of the two micronutrients, respectively. A study in young adults in Kampala( Reference Galukande, Jombwe and Fualal 77 ) and another in older adults in a rural setting( Reference Mugisha, Baisley and Asiki 78 ) found that folate deficiency was not common. Future studies need to consider vitamin A and vitamin B12 deficiencies. A second limitation of the study is that we were not able to assess gestational age by ultrasonography, which is considered the gold standard before 20 weeks’ gestation. Hoffman et al.( Reference Hoffman, Messer and Mendola 79 ) suggest that gestational age dating using the last menstrual period collected early in pregnancy approximates reasonably to the gestational age obtained from first-trimester ultrasound given the shorter period of time over which the woman has to recall. Our participants presented themselves for antenatal care early during pregnancy (as opposed to during the second or third trimesters which is the norm in our setting), thus there is reason to believe that their recall was fairly accurate. Moreover, the last menstrual period tends to overestimate the true gestational age( Reference Hoffman, Messer and Mendola 79 , Reference Geerts, Poggenpoel and Theron 80 ) and thus it is not likely that the women were over 16 weeks’ gestational age.
Conclusion
In conclusion, infections and/or inflammation, defined as elevated CRP or AGP concentrations, are important in the aetiology of anaemia in pregnant women during the first trimester in our setting. Public health measures to control infections and inflammation are urgently needed, in particular during pregnancy, as part of the anaemia control package. Considering that pregnancy triggers a state of inflammation and that CRP concentrations vary by race, there is a need to develop CRP reference values in normal, healthy African pregnant women in order to facilitate the interpretation of ferritin and other factors that are influenced by the acute-phase response. Further studies of the regulation of hepcidin in pregnancy in the presence of infection and/or inflammation are necessary in order to broaden our understanding of the overall regulation of Fe metabolism.
Acknowledgements
Acknowledgements: The women who took part in this study are gratefully acknowledged. The support of the Management, Kawempe Health Centre, the staff of the antenatal care clinic and of the laboratory, particularly Andrew Bamulumbye, is sincerely appreciated. The authors thank Pross Nammande for research assistance during data collection, Erwin Wiegerinck for the measurements of serum hepcidin and Levi Mugenyi for carrying out the data analysis. Financial support: This work was supported by Nutricia Research Foundation (grant number 2008-08) and a PhD Fellowship to R.K.B. from Innovations @ Makerere Committee. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: R.K.B., J.K.E. and L.D. designed the study. R.K.B. collected and entered data and carried out laboratory analyses for ferritin, transferrin receptor, CRP, AGP and H. pylori. H.T. and D.W.S. were responsible for hepcidin analysis and interpretation of results. R.K.B. drafted the manuscript. All others reviewed the drafts and gave final approval for submission. Ethics of human subject participation: The study was approved by Makerere University Faculty of Medicine Research and Ethics Committee and was registered with the Uganda National Council for Science and Technology.









