Dietary PUFA principally consist of linoleic (18 : 2n-6; LA) and α-linolenic acid (18 : 3n-3; ALA). In the land-based food chain, there are also small quantities present of arachidonic acid (20 : 4n-6), EPA (20 : 5n-3), docosapentaenoic acid n-3 (22 : 5n-3) and DHA (22 : 6n-3), but these are not routinely recorded in tables of food composition, which normally only report fatty acids to the nearest 0·1/100 g food. The relative abundance of the n-6 and n-3 metabolites in meat, eggs and dairy foods is influenced by the relative amounts of LA/ALA as well as the total amount of PUFA in the diet( 1 ). The land-based food chain, which is dominated by seeds and nuts, is LA rich. Indeed, many vegetable oils that are derived from oil-seeds predominantly supply most of the LA in the diet and very little ALA (<1 %). However, there are exceptions to this rule: soyabean and rapeseed oil contain about 7 and 10 % ALA and even greater amounts are found in flaxseed and hempseed oil. The membrane lipids of chloroplasts are rich in ALA and even though the fat contribution of green leafy vegetables to human diets is miniscule, they do contribute significantly to the dietary intake of herbivores. The body fat and milk from non-ruminant grass-fed herbivores contain relatively high amounts of ALA. However, nearly all the PUFA consumed by ruminants undergoes fermentation by rumen microbiota to stearic acid and an isomeric mixture of octadecenoic acids. Beef, lamb and dairy fat usually only contain 2–3 wt.% fatty acids as PUFA. However, cows fed on grass-based forages produce milk with a higher content of ALA than those fed on cereals( Reference Larsen, Nielsen and Butler 2 ). A few seed oils such as evening primrose (Oenothera biennis), borage (Boragio officinalis), blackcurrant seed (Ribes nigrum) and hempseed (Cannibis sativa) oils contain significant amounts of γ-linolenic acid (18 : 3n-3) and stearodonic acid (18 : 4n-3), but these fatty acids are generally not present in human diets unless taken as a dietary supplement. In addition, trace amounts of conjugated LA (9-cis, 11-trans 18 : 2 and 10-trans, 12-cis 18 : 2) and conjugated derivatives of ALA (9-cis, 11-trans, 15-cis 18 : 3 and 9-cis, 13-cis, 15-cis 18 : 3) are provided by fat from ruminant animals.
Marine lipids contain substantial amounts of stearodonic, EPA, docosapentaenoic acid n-3 and DHA, which are derived from synthesis by marine algae not necessarily from ALA but from the polyketide synthase pathway( Reference Metz, Roessler and Facciotti 3 ). The long-chain n-3 PUFA accumulate up the food chain with the highest dietary concentrations being found in the oily fish at the top of the food chain such as mackerel, tuna and salmon. Some marine algae such as Schizochytrium spp. also synthesise significant amounts of docosapentaenoic acid n-6 (22 : 5n-6). Oil (DHASCO-S™) from this species is used in dietary supplements and some fortified foods.
Assessment of dietary intakes for epidemiological purposes
Estimating dietary intake of PUFA from food tables is fraught with difficulties because the values are missing for many foods. The composition of fats in compound foods may also change with market prices; the so-called ‘blend flexibility’. Most prospective cohort studies have used FFQ to estimate intake of PUFA. However, these have a tendency to underestimate the intake of PUFA. Fortunately, there are good biomarkers of PUFA intake( Reference Hodson, Skeaff and Fielding 4 ) and the proportions of LA and ALA in adipose tissue or plasma lipids are relatively reliable indicators of past dietary intake and are probably superior to estimates from FFQ. The consumption of fish, especially oily fish, is often a good proxy for the intake of long-chain n-3 PUFA. Plasma and erythrocyte lipids are good indices of intake of EPA and DHA but erythrocyte phospholipids are poor indicators of ALA intake( Reference Sanders, Lewis and Slaughter 5 ) owing to the relatively low abundance of ALA in erythrocyte phospholipids.
A large number of studies have evaluated the effects of different types and levels of PUFA on risk factors for disease. However, translating changes into disease risk is complicated because sometimes factors are changed in opposing directions. Direct relationships in observational studies are subject to confounding by other non-dietary factors such as smoking habits and socioeconomic status. Identifying the threshold levels of intake required to elicit an effect is important because intakes below the threshold intake are unlikely to explain diet–disease relationships. For example, the threshold effect for the serum TAG lowering effects of EPA and DHA is about 1·5 g/d( Reference Harris 6 , Reference Sanders, Hall and Maniou 7 ) and higher intakes (3–6 g/d) to have blood pressure lowering, anti-inflammatory and antiothrombotic effects( 1 ). The levels of intakes seen in Northern European and North American populations are well below 1 g/d which is below the threshold dose for these effects. Recent large randomised controlled trials( Reference Sanders, Hall and Maniou 7 , Reference Singhal, Lanigan and Storry 8 ) have failed to show that lower intakes of EPA/DHA in the range 0·45–1·8 g/d have any effect on blood pressure or endothelial function.
Evidence using clinical endpoints from prospective cohort studies
A meta-analysis( Reference Mensink, Zock and Kester 9 ) of the effects of PUFA on blood lipids indicates that when 5 % of energy as SFA are replaced with PUFA, the total cholesterol:HDL ratio is lowered by about 0·2. Assuming that each 1·33 unit changes in the total cholesterol to HDL-cholesterol ratio are associated with a 33 % lower risk of fatal CHD( Reference Lewington, Whitlock and Clarke 10 ), this difference would translate into no more than a 4 % change in risk if the effects of PUFA were entirely explained by the effects on serum lipids. Jakobsen et al.( Reference Jakobsen, O'Reilly and Heitmann 11 ) conducted a meta-analysis of the pooled data from cohort studies and estimated that replacement of 5 % SFA with PUFA lowered CHD mortality by closer to 12 %. The analysis of LA intake in the Nurses Health Study suggested a linear reduction in risk of CHD with increasing intakes of up to about 8 % energy intake with no suggestion of adverse effects on CHD mortality( Reference Willett 12 ). However, no relationship was found between LA intake and CHD risk in the Zutphen Study in Holland( Reference de Goede, Geleijnse and Boer 13 ). Data based on the analysis of plasma lipids have found that higher compared with lower proportions of LA in plasma lipids are associated with a lower risk of CHD( Reference Harris, Poston and Haddock 14 , Reference Khaw, Friesen and Riboli 15 ). Fig. 1 illustrates the lower risk of CHD in the EPIC-Norfolk cohort( Reference Khaw, Friesen and Riboli 15 ) with lower levels of LA in phospholipids being associated with greater risk. It is to be noted that the intake of SFA has very little influence on their proportions in plasma phospholipids and so the association of a lower PUFA:SFA ratio in plasma phospholipids and CHD is unlikely to be due to a higher intake of SFA. When the intake of PUFA is low, there is increased production of palmitoleic acid (16 : 1n-7); most studies have found a higher proportion of palmitoleic acid in plasma and adipose tissue to be associated with increased risk, which would be consistent with the view that low intakes of PUFA are associated with increased risk.

Fig. 1. Risk of incident CHD in the EPIC Norfolk cohort estimated from a comparison of top and bottom quartiles of PUFA in plasma phospholipids. Data are taken from reference(Reference Khaw, Friesen and Riboli15) and show OR with 95 % CI adjusted for age, gender, social class, education, plasma vitamin C, blood pressure and serum cholesterol comparing the highest with the lowest quartile for 1591 cases and 2246 controls.
While the evidence indicates that just eating fish once weekly is associated with a 16 % lower risk of death from CHD( Reference Zheng, Huang and Yu 16 ), the relationship is subject to confounding (i.e. social class, Christian religious adherence which advocates eating fish on Friday etc.). Furthermore, the relationship is not confined to oily fish and also applies to lean fish. Fish also contains other nutrients besides long-chain n-3 PUFA in high abundance such as vitamin D, iodine and selenium, and depending on the species and where it is caught, may contain toxic metals (cadmium, mercury and arsenic) and organic contaminants (polychlorinated biphenyls and brominated fire retardants). Consequently, the health benefits associated with fish consumption may also depend on the provenance of the fish. As the intake of long-chain n-3 PUFA is primarily determined by the intake of oily fish, it is difficult to disentangle relationships with other nutrients/compounds that are supplied by fish( Reference Chowdhury, Stevens and Gorman 17 ).
A meta-analysis( Reference Pan, Chen and Chowdhury 18 ) using dietary intake of ALA reduced risk of CHD by 10 % and when combined with estimates using biomarkers the reduction was greater at 14 %. The size of this effect is comparable with the reduction in CVD mortality reported with increased intake of long-chain n-3 PUFA provided by fish. A recent long-term follow-up of an elderly cohort( Reference Mozaffarian, Lemaitre and King 19 ) found the proportions of EPA and DHA in plasma lipids of men in their seventies to be strongly predictive of improved survival and decreased risk of CVD death, a finding in agreement with the earlier report by Anderson et al. ( Reference Anderson, Sanders and Cruickshank 20 ). However, Khaw et al.( Reference Khaw, Friesen and Riboli 15 ) found no association with plasma phospholipid ALA, EPA and DHA with CVD risk in the Norfolk EPIC study. However, that study contained a significant number of vegetarians( Reference Welch, Shakya-Shrestha and Lentjes 21 ) who have low proportions of EPA and DHA but low risk factors for CHD, which may have masked any relationship.
There were no consistent associations between common sites of cancer and PUFA intake with the possible exception of prostate cancer where some studies have found a weak positive association with n-3 PUFA( Reference Dahm, Gorst-Rasmussen and Crowe 22 ). A nested case–control study from the Framingham cohort study found that the proportion of EPA and DHA in plasma phospholipids was associated with a low risk of developing Alzheimer's disease( Reference Schaefer, Bongard and Beiser 23 ). A meta-analysis suggests that low levels of long-chain n-3 PUFA are associated with increased risk of dementia( Reference Lin, Chiu and Huang 24 ).
Randomised controlled trials with clinical endpoints
CVD: replacing SFA with vegetable oils rich in linoleic acid
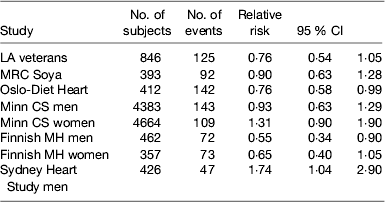
Several randomised controlled trials were conducted mainly in the 1960–1970s to evaluate the effect of partially replacing SFA with PUFA on preventing CHD. A major limitation of these early studies was the relatively small sample size and the short duration of follow-up as well as the relatively modest changes in serum cholesterol obtained. It is generally necessary to have at least 2 years of follow-up to assess the progression of atherosclerosis and much larger sample sizes are needed for primary prevention than secondary prevention because of the relatively low incidence rate. Hooper et al. in a Cochrane review( Reference Hooper, Summerbell and Thompson 25 ) of dietary interventions concluded that modification of fat intake results in a significant 14 % (95% CI 4, 23) reduction in CHD incidence and a non-significant 7 % reduction in cardiovascular mortality especially when serum cholesterol was lowered. However, there was a suggestion from the non-significant 23 % reduction in risk for an even better outcome when fat was both reduced and modified. Mozaffarian et al.( Reference Mozaffarian, Micha and Wallace 26 ) published a meta-analysis of the trials where PUFA had replaced SFA, but they included the Finnish Mental Hospital Study, which was not a randomised controlled trial but a trial of two diets in separate mental hospitals and which was excluded from the Cochrane review. This analysis suggested that the replacement of SFA with PUFA, mainly from soyabean and/or maize oil, which are both rich in LA, decreased the relative risk of CHD incidence by 17 %. A recently published re-analysis of the Sidney Heart Study conducted in the 1960s has re-ignited a controversy by suggesting that LA may have harmful effects on CHD mortality. This trial involved replacing butter with a safflower oil-based margarine and replaced other fats with safflower oil. Safflower oil is very high in LA (75 %) with only traces of linolenic acid and commercially available safflower oil margarine on sale in Australia at the time contained about 15 % trans-fatty acids. The study was terminated prematurely because of an adverse outcome on the intervention and was not published. Ramsden et al. ( Reference Ramsden, Zamora and Leelarthaepin 27 ) accessed the original trial data and by using modern statistical techniques reported a hazard ratio of 1·74 (95 % CI 1·04, 2·96) on the intervention diet compared with the control group which just achieved statistical significance at P=0·04. The limitations of this study are the small sample size and the very high proportion of younger men who died that were heavy smokers. This study was conducted at a time when the importance of smoking cessation in prevention of CHD was not widely accepted: 35/237 men died in the intervention group over the 5-year period v. 23/221 in the intervention group; sixteen of the deaths in the intervention group occurred in the first year of follow-up. The authors argued that the trials demonstrating benefits of PUFA had used mixtures of LA and linolenic acid or fish oils. However, their inclusion of the Los Angeles Veterans Study among those trials showing benefit is questionable as the intervention was based mainly on using maize oil and its main effect was to increase LA intake. Furthermore, including the Sydney Heart Study does not alter the conclusions of the previous meta-analyses (Table 1) and certainly did not warrant the call for a re-appraisal of LA intakes in modern diets as was made in the paper. However, it would be prudent to ensure that PUFA intake contains an appropriate balance of n-6 and n-3 PUFA and that the intake of trans-fatty acids is kept to a minimum.
Table 1. Studies comparing replacement of saturated fatty acid with PUFA on CHD incidence
Primary prevention trials are unlikely to be conducted in the future to answer this question because in order to detect a 5 % difference in incidence when the incidence is about 10/1000 would require studying 120 000 subjects in each arm for 5 years. Statin trials have proved far more effective in lowering blood cholesterol than dietary fat modification and so funding for any dietary trial is unlikely to be forthcoming.
CVD: increased intake of n-3 PUFA
The UK DART study( Reference Burr, Fehily and Gilbert 28 ) found that advice to eat two portions of oily fish/week resulted in a 29 % fall in mortality rate over a 2-year period but did not affect CHD incidence suggesting a fall in case fatality. However, a second study (DART-2) by the same group( Reference Burr, Ashfield-Watt and Dunstan 29 ), which used fish oil supplements instead of fish in patients with stable angina, found no benefit and a trend (P=0·06) for a less favourable outcome in the supplemented group. The GISSI investigators in two open label studies( Reference Marchioli, Barzi and Bomba 30 , Reference Tavazzi, Maggioni and Marchioli 31 ) reported reduced cardiovascular mortality with a purified fish oil TAG concentrate providing about 0·8 g EPA/DHA but a more recent study by the same group using a double-blind protocol found no effect( Reference Roncaglioni, Tombesi and Avanzini 32 ). Another open label study conducted in Japan which used 1·8 g ethyl EPA in addition to a statin v. a statin alone found a 19 % reduction in incident CHD in a mainly female population( Reference Yokoyama, Origasa and Matsuzaki 33 ). More recent larger double-blind controlled trials of EPA+DHA supplementation show no benefit on CVD incidence or mortality( Reference Kromhout, Giltay and Geleijnse 34 – Reference Mozaffarian, Marchioli and Macchia 36 ). These trials have been subject to meta-analyses( Reference Rizos, Ntzani and Bika 37 ). It was concluded that there was no evidence of a reduction in total mortality or CVD events with n-3 supplementation but there was a trend for cardiac death to be 9 % lower (95 % CI 85, 98) but the probability did not meet the level set (P=0·0063) by the authors, in order to allow for multiple endpoints. Only one sufficiently powered study (the Alpha–Omega trial) has compared EPA/DHA with ALA supplementation in secondary prevention and this showed no difference in the primary or the secondary outcomes( Reference Kromhout, Giltay and Geleijnse 34 ). The present evidence does not support the use of long-chain n-3 PUFA supplements for the secondary prevention of CHD. It should be noted that in the more recent trials, blood pressure and lipids were well controlled by medication, such as statins, and this may have obscured any dietary effect.
The Mediterranean diet study by de Lorgeril( Reference de Lorgeril, Salen and Martin 38 ) is often cited as evidence showing that an increased intake of ALA reduced CVD mortality, but this was a multifactorial intervention. The Indo-Mediterranean diet also claimed a beneficial effect of ALA but it emerged that this was fraudulent so its findings should be ignored( Reference Horton 39 ). The PREDIAMED study( Reference Estruch, Ros and Salas-Salvado 40 ) was conducted in over 7447 men and women (aged 55–80 years) who had not had a cardiovascular event in different regions of Spain. The trial compared a Mediterranean diet with virgin olive oil with or without walnuts, which are rich in ALA, compared with dietary advice based on the American Heart Association guidelines. The Mediterranean dietary advice also included an increased intake of fish, fruit and vegetables. The trial was stopped early because there was evidence of clear benefit in terms of the specified endpoint (major cardiovascular events) with most of the reduction being due to lower stroke mortality in the two groups allocated to the Mediterranean style diet but there was no difference between the virgin olive oil or the virgin olive + walnut group. Dietary advice for primary prevention of CHD using n-3 PUFA supplements is not likely to be ever put to test because of the very large numbers needed to demonstrate any effect and the growing evidence that changes in overall dietary pattern may be a more effective public health measure.
Cancer
Pilot studies based on the anti-cachexic effect of EPA in mice with cancer suggested that high doses of EPA might be of value in the management of cancer cachexia. However, despite what appeared to be a promising approach, the results from the phase 2 trial were equivocal( Reference Fearon, Von Meyenfeldt and Moses 41 ).
Inflammatory disorders
High intakes of EPA and DHA were shown to decrease cytokine concentrations and early clinical trials suggested that high intakes of EPA and DHA might be of value in the management of IgA nephropathy but follow-up studies failed to confirm these findings( Reference Liu and Wang 42 ). The same has been true regarding the use of n-3 fatty acids in the management of Crohn's disease( Reference Cabre, Manosa and Gassull 43 ). Some mild relief has been noted with high intake (>2·7 g/d) of long-chain n-3 PUFA on the symptoms of rheumatoid arthritis and related conditions such as systemic lupus erythrematosis but the effects have not been sufficient to warrant their use in clinical practice( Reference Lee, Bae and Song 44 ).
Psychiatric disorders
There currently is a lack of evidence to support the use of long-chain n-3 PUFA in the treatment of psychiatric disorders such as depression and a systematic review noted that the data available showed high risk of publication bias( Reference Bloch and Hannestad 45 ). However, this is a complex area that requires further research and may need to consider the long-term effects of early life nutrition since the brain is most vulnerable to the influences of PUFA intakes during fetal development and infancy that may have life-long effects( 46 ). The OPAL study evaluated the effect of supplementation with long-chain n-3 PUFA in cognitive function in elderly men and women over a 2-year period and was unable to detect any effect on cognitive decline( Reference Dangour, Allen and Elbourne 47 ). However, much longer-term studies may be needed to show any effects on cognitive decline.
Infant feeding and pregnancy
A large-number of randomised controlled trials of arachidonic and DHA supplementation of infant formula have been conducted on subjects of Cochrane reviews( Reference Simmer, Schulzke and Patole 48 , Reference Simmer, Patole and Rao 49 ). There is no clear evidence of benefit in term infants but a suggestion of improved visual function in pre-term infants. Neither is there any clear evidence of the benefit of supplementation with DHA in pregnancy on developmental outcomes, particularly as two of the more highly cited trials are at risk of bias( Reference Gould, Smithers and Makrides 50 ). There is limited data available on long-chain n-3 PUFA supplementation on pregnancy and other outcomes but one relatively large well-controlled study( Reference Palmer, Sullivan and Gold 51 ) provides tentative evidence for decreased incidence of atopic eczema but no effect on the primary outcome which is a reduction in the overall incidence of IgE associated allergies in the first year of life.
Recent dietary guidelines and current intakes
The UK recommendations on fatty acid intakes are based on the COMA report on Diet and CVD( 52 ) published in 1994 and a subsequent review of fish intake( 53 ). WHO/FAO( 1 ) reviewed the evidence and on the basis of the data from the prospective cohort studies concluded that there was convincing evidence that replacing SFA with PUFA decreases risk of CHD and suggested average macronutrient distribution ranges. The Institute of Medicine reviewed the Dietary Guidelines for Americans( 54 ) but did not set dietary reference values for PUFA. The European Food Safety Authority has set dietary reference values( 55 ). These are shown in Table 2.
Table 2. Dietary reference intakes for PUFA
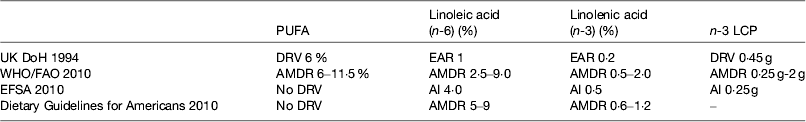
LCP, long-chain n-3 PUFA; DRV, dietary reference values; EAR, estimated average requirement; AMDR, acceptable macronutrient distribution range; AI, acceptable intake.
Intakes of PUFA have increased markedly in the UK over the past 40 years and current UK intakes are in the range of 5–6 % energy compared with 3–4 % energy in the 1970s. The UK National Dietary and Nutritional Survey( Reference Hoare, Bates, Prentice, Birch, Swan and Faron 56 ) is of limited value in providing information regarding the intake of PUFA in the UK diet owing in part to the paucity of information on fatty acid compositional data in the UK food composition database. Earlier data from the National Food Survey showed a remarkable change in the types of fats used in households; vegetable oils have largely replaced animal fats for cooking and there has been a fall in butter consumption and other yellow-fat spreads (margarine and low-fat spreads). For reasons that remain uncertain CHD incidence and mortality has fallen markedly in the UK by about 50 % in the last decade across all regions( Reference Smolina, Wright and Rayner 57 ). There appears to be little scope for further increasing the intake of PUFA, which is close to the 6 % target. More recently, new varieties of sunflower seed and soyabean oil have been introduced that have a lower content of LA and more oleic acid. Table 3 shows the effect on the overall diet based on analysis of 3-d duplicate diets where a high oleic sunflower oil is replaced by rapeseed oil and where the type of spreading fat is changed from a high LA blend to one with a lower level of linolenic acid with or without an increased intake of oily fish (2 servings/wk)( Reference Sanders, Lewis and Slaughter 5 ).
Table 3. Dietary intakes of PUFA determined by analysis of 3-d duplicate food intakes where the composition of dietary fats, spreads and fish intake were modified

LCP, long-chain n-3 PUFA 20 : 5n-3, 22 : 5n-3 and 22 : 6n-3.
Data from the OPTILIP study( Reference Sanders, Lewis and Slaughter 5 ).
Conclusion
Prospective cohort studies show consistent evidence that replacing SFA with PUFA lowers the risk of CHD. The results of the limited trials of dietary intervention in prevention of CHD are supportive of partial replacement of SFA with PUFA. The intake of both ALA and long-chain n-3 PUFA appears to be associated with lower risk of CHD. It is possible that changes in the food supply, particularly the wide-scale use of rapeseed oil, may have contributed to the decline in CHD. While early studies suggested a role for long-chain n-3 supplements in the secondary prevention of CHD, the more recent studies demonstrate no benefit. A balanced intake of PUFA can be obtained by consuming ordinary everyday foods without resorting to dietary supplements.
Acknowledgements
The author is grateful to Dr Maryam Al-Hilal for help in preparation of this article.
Financial support
The author acknowledges support from the Food Standards Agency and the Department of Health that have supported some of the research reported here. The Food Standards Agency and the Department of Health played no role in the design, analysis or writing of this article.
Conflicts of interest
None.






