Chronic constipation is a functional gastrointestinal disorder characterised by persistently difficult, infrequent or incomplete defaecation that affects approximately 14 % of the general population(Reference Mearin, Lacy and Chang1,Reference Suares and Ford2) . It may be diagnosed using symptom-based diagnostic criteria, such as the Rome IV criteria, according to which a diagnosis is made when two or more of the following symptoms are present for at least a quarter of bowel movements: hard or lumpy stools, straining, a sense of incomplete evacuation, use of manual manoeuvres to pass stool and a sense of anorectal obstruction(Reference Mearin, Lacy and Chang1). Nevertheless, both the general population and some doctors consider various other symptoms important for a diagnosis of constipation, including spending a long time on the toilet without achieving a bowel movement(Reference Dimidi, Cox and Grant3,Reference Dimidi, Dibley and Cotterill4) . Furthermore, a large cross-sectional survey in over 3000 members of the general population and doctors revealed differences in the symptoms considered important for a diagnosis of constipation between the general population, general practitioners (GP) and gastroenterology specialists, and that there was imperfect agreement with the Rome IV criteria, highlighting the difficulties and variability in the diagnosis of chronic constipation(Reference Dimidi, Cox and Grant3,Reference Dimidi, Dibley and Cotterill4) .
However chronic constipation is diagnosed, it impacts on people's lives, with straining, bloating, abdominal discomfort, abdominal pain and spending a long time on the toilet without a bowel movement being the symptoms most commonly described as being burdensome(Reference Dimidi, Cox and Grant3,Reference Wald, Scarpignato and Kamm5,Reference Sun, Dibonaventura and Purayidathil6) . A multinational survey has shown a negative correlation between the total number of symptoms of constipation experienced and quality of life (QoL), and lower health-related QoL has been reported by women, as well as by those under psychological stress, such as unemployment(Reference Wald, Scarpignato and Kamm5). Chronic constipation also impacts on work productivity(Reference Sun, Dibonaventura and Purayidathil6).
The high prevalence of constipation, its chronicity and its impact on QoL contribute to the utilisation of significant healthcare resources. The direct annual cost associated with the management of constipation has been shown to range from $1912 to $7522 annually per patient in the USA(Reference Nellesen, Yee and Chawla7), whilst in the UK, there are more than 1 million GP consultations and 69 054 hospital admissions annually where constipation is a diagnosis(8,Reference Shafe, Lee and Dalrymple9) . Treatment failure for constipation is also associated with a total incremental cost of $2978, with 60 % being spent on medical service costs(Reference Guerin, Carson and Lewis10), highlighting the importance of early successful management.
A variety of different management options exist for constipation, ranging from dietary interventions (e.g. dietary fibre(Reference Christodoulides, Dimidi and Fragkos11)) and over-the-counter products (e.g. laxatives) to prescription drugs (e.g. serotonin receptor agonists), behavioural interventions (e.g. biofeedback) and different surgical options(Reference Bharucha, Pemberton and Locke12). However, patient satisfaction is variable; for example, 49 % of patients initiating over-the-counter therapies and 58 % of patients initiating prescription therapies experience failure of that treatment(Reference Guerin, Carson and Lewis10). Another study reported that almost half of respondents were not completely satisfied with their current constipation treatment(Reference Johanson and Kralstein13). The reasons for patient dissatisfaction were mainly related to efficacy and safety, as well as cost issues and inconsistent results. These findings are supported by another recent web survey demonstrating that 17 % of patients with constipation were dissatisfied with laxative use(Reference Emmanuel, Quigley and Simren14). Taken together, these results show that there is still a substantial unmet need for new effective therapeutic strategies that would be appealing and satisfactory for people with constipation.
Over the past decade there has been an increase in research investigating the effect of probiotics on chronic constipation as a potential alternative management strategy. This review aims to assess and present evidence on the mechanisms of action of probiotics in constipation, their utilisation by patients and healthcare professionals and evidence for their effectiveness from clinical trials.
Potential mechanisms of action of probiotics in constipation
Probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host(Reference Hill, Guarner and Reid15). There are several mechanisms of action of probiotics relevant to constipation, including modulation of the gut microbiota and fermentation, nervous system and immune system, as shown in Fig. 1(Reference Dimidi, Christodoulides and Scott16).
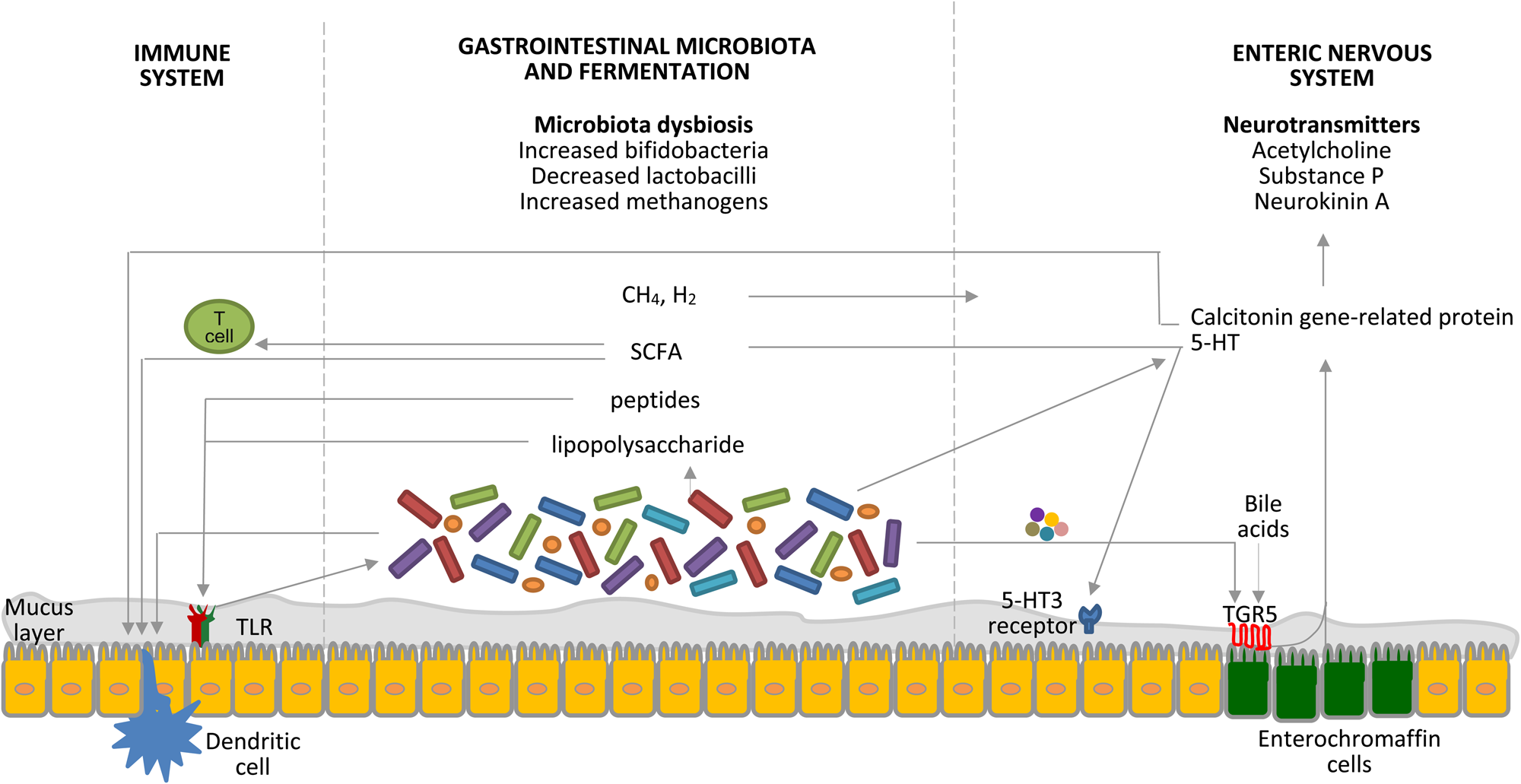
Fig. 1. (Colour online) Interrelated factors involved in the pathophysiology of constipation as potential targets for the therapeutic role of probiotics. Probiotics affect the gastrointestinal microbiota composition, the byproducts of which interact with pattern-recognition receptors, such as toll-like receptors (TLR), as well as with dendritic cells. SCFA increase intestinal regulatory T cells, which limit intestinal inflammation, by reducing histone deacetylase 9 gene expression(Reference Kieffer, Martin and Adams71). The gastrointestinal microbiota regulates 5-hydroxytryptamine (5-HT) production by elevating its synthesis by host enterochromaffin cells via the release of metabolites, such as deoxycholate, which activates TGR5, a G protein-coupled receptor, expressed by enterochromaffin cells(Reference Yano, Yu and Donaldson72). 5-HT is also released from enterochromaffin cells in response to SCFA produced by the gastrointestinal microbiota and stimulates 5-hydroxytryptamine type 3 (5-HT3) receptors located on the vagal afferent fibres, resulting in muscle contractions(Reference Fukumoto, Tatewaki and Yamada27). Gases produced by the gastrointestinal microbiota seem to affect gut motility via the enteric nervous system, rather than the brain–gut axis; however, the exact mechanisms are still unknown(Reference Triantafyllou, Chang and Pimentel73). Moreover, the gastrointestinal microbiota is key to the development of the enteric nervous system, which is the primary regulator of gut motility, and certain bacteria are known to produce 5-HT. Calcitonin gene-related protein, a sensory neuropeptide, modulates dendritic cell function and may signal the presence of gastrointestinal microbiota to the brain(Reference Collins and Bercik74). Components of the gastrointestinal microbiota also act via intestinal dendritic cells to influence the inflammatory process(Reference Ng, Benjamin and McCarthy75). TLR signalling controls the enteric nervous system structure and neuromuscular function and hence motility(Reference Brun, Giron and Qesari76). Bile acids activate TGR5 expressed by enterochromaffin cells and myenteric neurons and release 5-HT and calcitonin gene-related peptide. Furthermore, probiotics appear to interact with the gut–brain axis via the modulation of afferent sensory nerves that influence gut motility. CH4, methane; H2, hydrogen. Taken with permission from Dimidi et al.(Reference Dimidi, Christodoulides and Scott16).
Several studies have demonstrated differences in the gut microbiota composition between people with and without constipation(Reference Dimidi, Christodoulides and Scott16), with a decreased concentration of bifidobacteria and lactobacilli, as well as increased numbers of Bacteroidetes, identified in people with constipation(Reference Dimidi, Christodoulides and Scott16–Reference Parthasarathy, Chen and Chen18). Faecal microbiota composition has been shown to correlate with colonic transit time, while the colonic mucosal microbiota composition correlates with constipation status(Reference Parthasarathy, Chen and Chen18). Although the impact of probiotics on the microbiota in constipation is not well understood, a small number of trials have demonstrated significant changes in gut microbiota composition following probiotic supplementation(Reference Dimidi, Christodoulides and Scott16). For example, supplementation of Bifidobacterium lactis GCL2505 or Lactobacillus casei Shirota increased the concentration of bifidobacteria, however B. lactis NCC2818 and VSL#3, a multi-strain probiotic, had no impact on gut microbiota composition(Reference Ishizuka, Tomizuka and Aoki19–Reference Dimidi, Zdanaviciene and Christodoulides22). These results suggest that administration of probiotics may impact on certain microbiota components, but it is yet to be determined what impact this change has on constipation, and whether effects are mediated through microbiota modification or other mechanisms.
It is likely that it is the physiologically active substances produced by the gut microbiota that have an impact on motility, rather than the microbiota per se. Metabolic byproducts of the microbiota that might contribute to a change in gut function in response to probiotic supplementation include SCFA, which are primary end-products of fermentation of non-digestible food components including carbohydrates (Fig. 1)(Reference Morrison and Preston23). In vitro and ex vivo experiments have shown that SCFA may affect gut motility by stimulating mucosal receptors connecting to enteric or vagal nerves(Reference Yajima24), acting directly on colonic smooth muscle(Reference Cherbut, Aube and Blottiere25,Reference Rondeau, Meltzer and Michel26) or via increasing intraluminal serotonin concentration, an excitatory neurotransmitter(Reference Fukumoto, Tatewaki and Yamada27). When investigating the impact of probiotics on SCFA concentrations in people with constipation, several human studies show significant changes(Reference Matsumoto, Takada and Shimizu21,Reference Liu, Xu and Han28–Reference Valerio, Russo and de Candia30) , however others show little impact(Reference Riezzo, Orlando and D'Attoma31,Reference Sakai, Makino and Ishikawa32) . These results may be attributed to the different strains used in the studies and because stool, rather than luminal SCFA concentrations are measured, which is not predictive of SCFA production in the proximal colon(Reference Marsono, Illman and Clarke33).
The colonic mucus may also play a role in regulating gut motility as it acts as a lubricant and facilitates stool passage(Reference Matsuo, Ota and Akamatsu34), while bile acids may affect motility through luminal electrolyte and water transport regulation as demonstrated by in vitro and animal studies(Reference Snape, Shiff and Cohen35,Reference Keely, Scharl and Bertelsen36) . However, there is currently much less evidence that probiotics affect bile acid metabolism or mucin excretion in human subjects(Reference Caballero-Franco, Keller and De Simone37–Reference Marteau, Cuillerier and Meance39).
Modulation of microbiota–gut–brain interactions with probiotics has been demonstrated in healthy people(Reference Tillisch, Labus and Kilpatrick40), while L. reuteri has been shown to increase the excitability of myenteric neurons in rats and interact with the gut–brain axis via alterations in afferent sensory nerves that affect gut motility, indicating that that probiotics do impact on the enteric nervous system(Reference Kunze, Mao and Wang41,Reference Wang, Mao and Diorio42) . Hence, probiotic-mediated modulation of microbiota–gut–brain interactions has been proposed as a potential novel therapeutic tool for the treatment of gut motility disorders, including constipation; however, there are no human studies in constipation.
Lastly, there is emerging evidence of an inflammatory response in some people with constipation(Reference Khalif, Quigley and Konovitch43), which may alter enteric sensory and motor function(Reference Collins44). A potential impact of the probiotics on inflammatory response may, therefore, potentially affect gut motility regulation and, hence, constipation. Indeed, certain probiotics modulate the mucosal immune barrier or systemic immune barrier, and normalise dysmotility(Reference Guarino, Altomare and Stasi45,Reference Isolauri, Sutas and Kankaanpaa46) . For example, L. paracasei has been shown to produce antagonistic metabolites and antioxidants, such as glutathionine, to stimulate the immune system in vitro (Reference Ibnou-Zekri, Blum and Schiffrin47), while people who consumed B. lactis for 6 weeks had significantly higher interferon-α, and polymorphonuclear cell phagocytic capacity compared to placebo(Reference Arunachalam, Gill and Chandra48). Hence, probiotics may have beneficial effects with regards to some components of the immune system that could potentially influence gut motility, but the effect on their immune regulation in constipation has yet to be extensively investigated.
Therefore, there is evidence that certain probiotics may confer beneficial effects on constipation via their impact on the gut microbiota and fermentation, the enteric and central nervous system, and the immune system. However, the vast majority of evidence originates from in vitro and animal studies and thus the mechanisms of action of probiotics in human subjects remain unclear and warrants further research.
Effectiveness of probiotics in constipation
The impact of probiotics on gut transit time (GTT) and the management of constipation have been investigated by many randomised controlled trials (RCT), as well as in systematic reviews and meta-analyses, and these have been performed mainly for the probiotics bifidobacteria and lactobacilli.
In terms of bifidobacteria, one study that investigated the effect of B. lactis DN-173010 revealed significant improvement in stool consistency, as well as an increase in stool frequency by +1·5 bowel movements per week, compared to placebo in 135 women with chronic constipation(Reference Yang, He and Hu49). Another triple-blind, three arm, placebo-controlled RCT that compared consumption of two different doses of B. lactis HN019 and placebo for 2 weeks in eighty-eight people with constipation showed that the probiotic significantly decreased whole GTT in a dose-dependent manner; the high dose probiotic group experienced a reduction of −28 h in whole GTT compared to −19 h decrease and +1 h increase in the low dose and placebo group respectively (P < 0·001)(Reference Waller, Gopal and Leyer50). Interestingly, a subsequent double-blind RCT that investigated the effect of the same B. lactis HN019 strain on 228 people with chronic constipation showed no significant differences in whole GTT, gut symptoms, constipation-related QoL, stool frequency or stool consistency between the probiotic and placebo groups(Reference Ibarra, Latreille-Barbier and Donazzolo51). Similarly, a double-blind placebo-controlled RCT investigating the effect of B. lactis NCC2818 on seventy-five people with chronic constipation showed no significant differences in whole and regional GTT, stool frequency, stool consistency, gut symptoms, QoL and stool microbiota composition(Reference Dimidi, Zdanaviciene and Christodoulides22). Therefore, differing results have been demonstrated even for different B. lactis strains, highlighting the effects of probiotics may be strain-specific.
In terms of lactobacilli, an RCT in twenty people with chronic constipation also showed a significant increase in stool frequency compared to controls following L. reuteri DSM 17938 administration, but no improvement in stool consistency(Reference Ojetti, Ianiro and Tortora52). L. casei Shirota has been shown to decrease the occurrence of hard stool compared to placebo in chronic constipation, while flatulence and bloating were unaffected(Reference Koebnick, Wagner and Leitzmann53). It is worth noting that both the probiotic and placebo groups experienced an increase in stool frequency by +3 and +2 bowel movements per week compared to baseline, respectively, even though this difference between the two groups was significant(Reference Koebnick, Wagner and Leitzmann53). Interestingly, an increase in stool frequency was also observed at baseline in both groups compared to the initial assessment which had taken place 2 weeks prior to baseline, indicating a possible placebo effect(Reference Koebnick, Wagner and Leitzmann53). Another RCT in ninety people with chronic constipation showed that 4 weeks of L. casei Shirota administration did not improve stool consistency and quantity compared to placebo; however, a significant within-group improvement was seen following the probiotic(Reference Mazlyn, Nagarajah and Fatimah54). A double-blind, three-arm RCT in 300 people with hard stools (but not specifically with a diagnosis of constipation) reported a significant improvement in stool frequency and consistency, ease of expulsion, sense of complete evacuation and bloating following the administration of L. plantarum LMG P-21021 and B. breve DSM 16604, or B. lactis LMG P-21384, compared to placebo(Reference Del Piano, Carmagnola and Anderloni55).
Six systematic reviews have investigated the effect of probiotics on outcomes relevant to chronic constipation, as summarised in Table 1. Of these systematic reviews, one did not synthesise data into a meta-analysis due to studies not being sufficiently similar and of sufficient quality(Reference Chmielewska and Szajewska56), and another(Reference Miller, Zimmermann and Ouwehand57) is similar to a subsequent systematic review published a year later by the same group(Reference Miller, Ouwehand and Ibarra58) and therefore both are summarised in the table but not discussed here. The findings of the remaining four systematic reviews are summarised below.
Table 1. Systematic reviews and meta-analyses of randomised controlled trials (RCT) investigating the effect of probiotics on gut transit time (GTT) and constipation in adults

BM, bowel movements; md, mean difference; smd, standard mean difference; SR, systematic review; SR/MA, systematic review and meta-analysis; RR, risk ratio; wk, week.
First, a systematic review and meta-analysis of eleven RCT (n 464) that assessed the effect of probiotics (including B. lactis, B. longum, L. casei and L. rhamnosus with doses ranging from 0·48 × 109 to 97·5 × 109 CFU/d and treatment duration from 10 to 28 d) on GTT in both healthy and constipated people was published in 2013 and revealed a significant decrease in GTT (standard mean difference (smd): 0·40, P < 0·001) following probiotic (median period of consumption: 18 d), with the presence of constipation being predictive of greater GTT reductions(Reference Miller and Ouwehand59); greater reductions in GTT were seen in people with constipation compared to those without constipation in a further sub-group analysis of seven studies (smd: 0·59, P = 0·01)(Reference Miller and Ouwehand59).
Secondly, in 2014, a systematic review and meta-analysis of two RCT (n 110) that administered 6·5 × 109 CFU/d L. casei Shirota for 3 weeks or 1·25 × 109 CFU/d B. lactis for 2 weeks showed a significant increase in stool frequency (mean difference: +1·5 bowel movements per week (95 % CI 1·0, 2·0) bowel movements per week), but there was no significant difference in the dichotomous outcome of failure to respond to therapy compared to placebo (risk ratio: 0·29, 95 % CI 0·07, 1·12)(Reference Ford, Quigley and Lacy60).
Thirdly, a systematic review and meta-analysis of fourteen RCT (n 1182) was also published in 2014 showing that probiotics significantly reduced whole GTT by −12·4 (95 % CI −22·3, −2·5) h and increased stool frequency by +1·3 bowel movements per week (95 % CI 0·7, 1·9) bowel movements per week)(Reference Dimidi, Christodoulides and Fragkos61). The dose of probiotics used in the individual studies ranged from 108 to 3 × 1010 CFU/d and the treatment period varied from 2 to 8 weeks. Importantly, the sensitivity analysis showed species- and strain-specific effects of probiotics as stool frequency was significantly higher following B. lactis species (mean difference: +1·5 bowel movements per week; 95 % CI 0·7, 2·3 bowel movements per week), but not following L. casei Shirota (mean difference: −0·2 bowel movements per week; 95 % CI −0·8, 0·9 bowel movements per week)(Reference Dimidi, Christodoulides and Fragkos61). Similarly, stool consistency was significantly improved following B. lactis administration, but not for L. casei Shirota(Reference Dimidi, Christodoulides and Fragkos61).
Fourthly, a recent systematic review and meta-analysis of twenty-one RCT (n 2656) showed that probiotics significantly reduced GTT (smd: 0·65, P < 0·001) in people with constipation, and the mean difference in stool frequency was +0·83 bowel movements per week (P < 0·001); however, after adjusting for publication bias, the difference in stool frequency was reduced to 0·3 bowel movements per week (95 % CI −0·01, 0·62 bowel movements per week) which was not statistically significant(Reference Miller, Ouwehand and Ibarra58). The dose of probiotics used in the individual studies ranged from 0·1 × 109 to 30 × 109 CFU/d and the treatment period varied from 7 to 84 d. In addition, the probiotic products used in some of the studies also contained additional ingredients (e.g. psyllium, inulin and fructo-oligosaccharides) that did not allow for the effect of the probiotic alone to be isolated(Reference Miller, Ouwehand and Ibarra58). This, in addition to the increased heterogeneity among the studies, denotes that caution is needed in interpreting the results.
The interpretation of these findings from systematic reviews and meta-analyses is challenging due to high heterogeneity and risk of bias of the individual studies, and because species- and strain-specific effects have been identified. First, although meta-analyses synthesise data from many trials in order to improve the statistical power to detect the direction, size and consistency of a clinical effect, they cannot overcome limitations in the design of individual trials. Secondly, different probiotic species and strains have different microbiological and physiological characteristics, and therefore synthesising data from different probiotics and different doses is questionable(Reference Whelan62). Despite these challenges, the results provide cautious optimism for the recommendation of specific probiotic strains in the management of chronic constipation. Further adequately powered RCT using standardised outcome measures are needed to determine which species/strains, doses and duration of probiotics are efficacious.
Use of probiotics in constipation
Given the impact of constipation on QoL, and the effectiveness of certain probiotics on improving constipation-related symptoms, there is increasing interest in using probiotics as a therapeutic option.
A survey in 269 patients attending outpatient gastroenterology clinics identified that 44 % used complementary and alternative medicines, with constipation being the most cited symptom to be addressed, and probiotics being the most common complementary and alternative medicine used(Reference Hung, Kang and Bollom63).
The prevalence of probiotic use in constipation was also confirmed in a recent large cross-sectional study in 2557 members of the UK general population, of whom 1623 had self-reported constipation(Reference Dimidi, Cox and Scott64). This study revealed that the strongest predictors for probiotic use in the general population were having constipation, although this was a population selected for such symptoms(Reference Dimidi, Cox and Scott64). It was also shown that 60 % of the general population with constipation had previously used or were currently using probiotics, compared to 51 % of those without constipation (P < 0·001). In fact, self-reported constipation was associated with a 4·7 greater likelihood of current probiotic use (OR: 4·7, 95 % CI 3·8, 5·7, P < 0·001). In those with self-reported constipation, significant predictors of probiotic use for either general health or gut health specifically was ‘believing probiotics have been tested in research for their effectiveness on constipation’ (OR 2·06, 95 % CI 1·56, 2·72, P < 0·001), having a university degree (OR: 1·76, 95 % CI 1·32, 2·35, P < 0·001), being older (OR: 1·02, 95 % CI 1·01, 1·03, P < 0·001), and being female (OR: 0·54, 95 % CI 0·35, 0·81, P = 0·003)(Reference Dimidi, Cox and Scott64). The finding that females are more likely to use probiotics than males may be explained by the fact that constipated women report significantly worse QoL compared to constipated men(Reference Wald, Scarpignato and Kamm5). Therefore, women may be more likely to seek additional or alternative treatments for their symptoms than men. Indeed, a previous study has confirmed that constipated subjects seeking medical care are most likely to be females(Reference Cheng, Chan and Hui65).
In terms of the recommendation of probiotics by doctors, probiotics seem to be commonly recommended for the management of gastrointestinal disorders, such as chronic diarrhoea and irritable bowel syndrome(Reference Cordina, Shaikh and Shrestha66). A UK survey of over 1500 primary care health professionals (e.g. GP, dietitians, nurses) showed that 78 % of GP advise probiotic use for their patients, with constipation being the fifth most common condition for which they are recommended(Reference Jordan, Johnson and Thomas67). However, a recent survey in 411 GP and 365 gastroenterology specialists showed that 66 % of GP and 74 % of gastroenterology specialists do not recommend them for constipation(Reference Dimidi, Cox and Scott64). A possible reason for this might be the perceived lack of research evidence in this area. Indeed, only 35 % of GP and 43 % of gastroenterology specialists believe there is evidence for probiotic use in constipation(Reference Dimidi, Cox and Scott64), despite existing evidence from RCT showing that certain probiotic strains may improve constipation-related symptoms(Reference Miller, Ouwehand and Ibarra58,Reference Dimidi, Christodoulides and Fragkos61) . Interestingly, the gastroenterology specialists who believed there is evidence for probiotics in constipation thought probiotics were more effective for the management of constipation, compared with those who did not believe there is evidence(Reference Dimidi, Cox and Scott64). Belief in the existence of scientific evidence for probiotics among doctors is therefore likely an influencer on the belief in their impact on symptoms and on their behaviour in terms of recommending them to patients.
L. casei Shirota (Yakult) and a mixed preparation of Streptococcus, Bifidobacterium and Lactobacillus (VSL#3) are the probiotics most commonly recommended by gastroenterology specialists and GP for constipation, respectively, whereas B. lactis DN-173010 (Activia), L. casei DN 114 001 (Actimel) and L. casei Shirota (Yakult) are the probiotics most commonly used by the general population with constipation(Reference Dimidi, Cox and Scott64). This is in agreement with the probiotic products that patients with inflammatory bowel disease also choose to use(Reference Hedin, Mullard and Sharratt68). Although there are a few reports showing beneficial results of some of these strains in constipation, these studies have various limitations, such as small sample sizes or the absence of objective outcomes(Reference Kim, Choi and Park20,Reference Mazlyn, Nagarajah and Fatimah54,Reference He, Hu and Yang69,Reference Krammer, von Seggern and Schaumburg70) . Interestingly, no study has been previously published on the effect of Actimel (L. casei DN 114 001) on constipation. Therefore, the choice of the probiotic product used by the general population and doctors is not necessarily driven by the current scientific evidence available, but could be influenced by factors such as availability or product advertising.
Indeed, TV adverts were the most common source of information for probiotics in gut health, followed by family, friends and the internet in general (Fig. 2)(Reference Dimidi, Cox and Scott64). This is mostly in agreement with the findings of a previous survey that showed that commercial advertising was the most common source of information for probiotic use in patients with inflammatory bowel disease, followed by family and friends, and healthcare professionals(Reference Hedin, Mullard and Sharratt68). Similarly, another survey showed that the most common sources of information for the use of complementary and alternative medicines (including probiotics) in gastrointestinal conditions were family, newspapers, magazines, the internet and friends(Reference Hung, Kang and Bollom63).

Fig. 2. (Colour online) Sources of information for probiotic use for gut health in people with constipation based on an online survey in 346 people with self-reported constipation. GP, general practitioner (4·6 % did not report a source of information for probiotic use and this is not depicted in the figure). Adapted from data presented in Dimidi et al.(Reference Dimidi, Cox and Scott64).
Taken together, evidence shows that more people with self-reported constipation use probiotics compared to those without self-reported constipation, however, the vast majority of GP and gastroenterology specialists do not recommend them for constipation. This could possibly be explained by the fact that the vast majority of doctors do not believe probiotics have been tested in research studies for their effect on constipation.
Conclusion
Evidence on the effectiveness of probiotics remains varied, with certain strains exhibiting beneficial effects, while others show little effect. This highlights that the effects of probiotics may be strain-specific and that each strain needs to be tested in a high-quality RCT using standardised and validated assessment techniques in order to be able to devise clinical recommendations regarding probiotic use in constipation in the future. This, in combination with the increased probiotic usage in constipation, indicates a need to clearly communicate and raise the public's awareness on the current state of evidence on probiotics and constipation. Education of healthcare professionals is also required on both the strain-specificity of the effects of probiotics, but also on the degree of probiotic usage by the public; this may encourage healthcare professionals to query about probiotics and discuss their use with patients and, therefore, educate them on the uncertainty in the available evidence.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
E. D. has received an education grant from Alpro and research funding from the Almond Board of California, the International Nut and Dried Fruit Council and Nestec Ltd. M. S. has served as a speaker for Laborie, and has received research funding from the Medical Research Council UK, Nestec Ltd, Mui Scientific, Bowel & Cancer Research and the Almond Board of California. K. W. has served as a consultant for Danone, has received speaker fees from Alpro, has received research funding from Clasado Biosciences, Nestec Ltd, Almond Board of California and the International Nut and Dried Fruit Council, and receives royalties from FoodMaestro.
Authorship
E. D. drafted the manuscript. S. M. S. and K. W. reviewed the manuscript. All authors have approved the final version of the paper, including the authorship list.





