Dietary guidance to reduce total fat intakes might decrease harmful SFA consumption if implemented successfully; however, it will also reduce the supply of beneficial unsaturated fatty acids (FA), including n-3 fatty acids, currently considered inadequate. A more prudent approach might be to adjust the relative proportion of the various FA in our food aiming to increase PUFA intake and reduce SFA. On average in the UK between 53 and 57 % of our SFA intake comes from animal-derived foods; dairy, meat and eggs, yet their consumption is estimated to provide only 30 % of PUFA intake( Reference Pot, Prynne and Roberts 1 ). This fact, along with the known variation in FA profiles in animal-derived foods, makes them obvious candidates to investigate the scope to manipulate fat composition as a means of improving the balance of FA in our diet.
The benefits of PUFA over SFA are well recognised and the Food and Agriculture Organization paper 91 on fats and FA in human nutrition( 2 ) and the European Food Standards Agency( 3 ) provide relatively recent consensus on acceptable guidelines for fat intake and composition, although identifying knowledge gaps and the need for further research. If attempting to manipulate the fat content of animal-derived food to benefit our health the consensus appears to aim for: (a) reducing SFA, (b) increasing PUFA, (c) increase n-3 PUFA especially long-chain n-3 fatty acids such as EPA (20 : 5 n-3), docosapentaenoic acid (22 : 5 n-3) and DHA (22 : 6 n-3) rather than n-6 PUFA and hence reduce the n-6:n-3 ratio and (d) increase the conjugated linoleic acid (CLA) and possibly vaccenic acid (VA; trans-11-18 : 1) content of ruminant-derived foods( Reference Scollan, Hocquette and Nuernberg 4 – Reference Woods and Fearon 7 ). Activity to raise CLA and VA content is a relatively long-standing target for improvement despite the lack of official guidelines for their consumption. There are numerous studies showing the potential benefits from CLA in in vitro or animal models, suggesting activity against cancer, hypertension, diabetes and other conditions( Reference Bhattacharya, Banu and Rahman 8 , Reference Wahle, Heys and Rotondo 9 ) and evidence that it can be synthesised in adipose tissue from VA( Reference Turpeinen, Mutanen and Aro 10 ).
Fat composition of animal-derived foods
The PUFA content of animal-derived foods taken from the UK( Reference McCance and Widdowson 11 ) and US( 12 ) food composition tables are shown in Table 1, although such figures could be misleading considering the apparent lack of detectable longer-chain PUFA in many of these foods. Hopefully, the present paper will explain how variable PUFA profiles can be, what factors of their management lead to this variation and, if such tables are to be useful in this respect, why animal-derived foods may need to be classified according to production systems. Such variation may explain differences between the two datasets since some aspects of animal production do differ between the UK and the US.
Table 1. Fatty acid composition of animal-derived foods: UK( Reference McCance and Widdowson 11 ) and US( 12 ) values

What influences fatty acid profiles in animal-derived foods
The balance of FA in milk, meat and eggs depends on the fat profile of feeds the animals consume although it is also greatly influenced by their digestive physiology, as explained in a review by Woods and Fearon( Reference Woods and Fearon 7 ). The presence of a rumen (as in cattle and sheep) not only determines which feeds are appropriate for our livestock, but it also has a major effect on the nature of FA absorbed and ultimately secreted into milk or eggs or deposited in meat. Pigs and poultry have relatively simple digestive systems and absorb FA in more or less the same proportions as they are found in their diet. On the other hand, fat absorption by cattle and sheep is heavily influenced by rumen microbial activity that can hydrogenate (saturate) up to 95 % of dietary PUFA; hence the high levels of SFA in ruminant milk or meat and challenge to increase the PUFA content.
Livestock diets and fat profiles
Modern livestock diets are a combination of various plant substances and the FA supply can be quite variable depending on the ingredients; largely influenced by the relative proportion of leaves and seeds; with some notable exceptions, seeds tend to be high in n-6 and leaves in n-3. Many of the feeds used, along with their lipid content and FA profiles are shown in Table 2.
Table 2. Typical total lipid and individual fatty acid (FA) content of feedstuffs used in livestock diets

Ext., extracted.
Wild ancestors of pigs and poultry were omnivores consuming a range of invertebrates and plants although modern production systems tend to use feeds largely of plant origin and mostly seeds. Diets are generally cereal based using oilseed meals (after chemical or physical extraction of oil for human consumption), peas or beans to supply necessary protein, possibly with fishmeal to provide an adequate amino acid balance for young animals or those at a high level of production. As a general rule, cereals are relatively low (<30 g/kg) in lipid and dominated by an n-6 FA, linoleic acid (cis-9,12–18 : 2), as are many of the oilseed meals, with the exception of rapeseed meal with a high proportion of oleic acid (C18 : 1 c9) and linseed (α-linoleic acid (ALA), cis-9,12,15–18 : 3) although the latter is not routinely fed.
Cattle and sheep evolved from grazing herbivores with a diet of leafy vegetation, which is also relatively low in lipids although, in this case, dominated by ALA. The lipid content of these forages declines as plants mature, accumulating cellulose and hemi-cellulose cell walls that dilute the relative proportion of the phospholipids in cell and organelle membranes. The FA content of forage is also altered by conservation or preservation; drying or fermentation to prepare hay or silage will diminish their PUFA content. Owing to their evolution and digestive physiology, ruminants need a minimum inclusion of forage or fibrous feeds to maintain digestive health although most modern production systems deviate from solely forage-based diets. Growth rates and milk yield are enhanced by feeding supplementary cereals, oilseed meals, by-products from food and drink manufacture and occasionally additional lipid supplements. Intensive dairy cows may consume only 30 % of their diet as (conserved) forage and in some cases intensive beef and lamb diets may be devoid of forage and rely on animal browsing straw bedding to maintain rumen health.
Challenges and scope to change
There is considerable research into the manipulation of livestock dietary fat intakes as a means to change the FA profiles of the food they produce. This section will describe some activity and progress within three product ranges; eggs, dairy and beef. In the case of eggs, increasing the PUFA content of the diet has a direct influence over the FA profiles of the product while manipulating ruminant fat quality is a much less predictable science.
Eggs
With respect to offering consumer foods with enhanced fat profiles, eggs are possibly more advanced than other animal-derived foods since ‘Omega 3’ eggs, enriched with n-3 PUFA, have been on sale for some time. As mentioned, it is relatively straightforward to increase the PUFA content of egg yolk by increasing PUFA supply in the hens’ diet although there are still questions to be addressed in the relationship. Are resulting eggs (a) acceptable and (b) offering a health benefit to consumers? A recent review article on dietary enrichment of eggs with n-3 fatty acids Fraeye et al.( Reference Fraeye, Bruneel and Lemahieu 6 ) compares twenty-six studies using linseed, fish oils and/or micro-algae as the source of n-3 PUFA, conducted between 1991 and 2011. One striking feature of the studies covered in this review is the diversity in the PUFA content reported in eggs from hens on the control treatments, reflecting variability in what might be considered as baseline diets, with respect to both n-3 and n-6 supply. ALA content of yolks from hens on the control diets showed a 10–20-fold difference, ranging from 13 to 70 mg per egg (seven studies) or 0·1 to 1·21 % of total FA (twelve studies) and DHA content from 20 to 62 mg per egg (eight studies), 0·1 to 2·2 % of total FA (twelve studies) or 1·7 to 2·3 mg/g yolk (three studies).
Changes in egg composition as a result of supplementation are also extremely variable and reported to be influenced by many factors. Analysis of the data presented in this review paper( Reference Fraeye, Bruneel and Lemahieu 6 ) shows that concentrations of EPA in eggs vary little although the increase in ALA and DHA content is variable and significantly influenced by: (a) the type of supplement used (P < 0·01 and 0·05, respectively), (b) the level of dietary inclusion (P < 0·001) and (c) the content of ALA and DHA in eggs from the control diets (P < 0·01 and 0·001, respectively). The response to supplementation, especially with linseed feeding, was greater if the control diet produced eggs low in n-3. Fraeye et al.( Reference Fraeye, Bruneel and Lemahieu 6 ) suggest that dietary n-6 supply is also important as was the type or strain of hens, since their ability to convert ALA to longer chain n-3 varies with genetics and the age of the birds, as well as the relative competition between n-3 and n-6. Most studies covered in the present paper supplemented or substituted diets with linseed (high in ALA), fish oil (high in long-chain n-3 such as EPA and DHA) and/or heterotrophic micro algae (cultured ‘seaweeds’ also high in long-chain n-3 such as EPA and DHA), all of which appear to increase the n-3 content of eggs and reduce their n-6 concentrations. Fig. 1 shows the response in ALA and DHA content of eggs in the twenty-four comparisons where linseed was the sole supplement. This substantially increased the ALA content of egg yolk (up to a 27-fold rise) in proportion to feeding rate and also caused a smaller but more consistent (1·5–3·5-fold) rise in the DHA content of eggs, indicating hens’ ability (albeit limited) to elongate and desaturate dietary ALA. Fish oil inclusion gave a less dramatic or predictable response in eight examples, with a relatively small rise in ALA content of eggs (2·3-fold increase (se 0·8)) and a somewhat greater rise in DHA (almost 6-fold (se 1·6)), the magnitude of which appears to be influenced more by the control diets rather than rates of supplementation. Micro-algae was fed in ten comparisons giving responses similar to fish-oil, with a small increase in ALA (1-fold (se 0.1)) and a greater rise in DHA content of eggs (4-fold (se 0.5)).

Fig. 1. Changes in α-linolenic acid (no fill) and DHA (grey columns) content of egg yolk following linseed supplementation( Reference Fraeye, Bruneel and Lemahieu 6 ). Columns depict 2nd and 3rd percentiles, with median values and error bars representing the range.
Unfortunately, the effective increase in n-3 content of eggs is only part of the overall story; eggs produced from supplementation often have a ‘fishy taint’ unacceptable to consumers and the elevated PUFA content may makes them prone to oxidation, although Fraeye et al.( Reference Fraeye, Bruneel and Lemahieu 6 ) report micro-algae (especially when fed intact rather than by using an extracted lipid product) is less marked in these respects. In reality, moderate levels of linseed (<10 % of the diet) or the addition of <1·5 % of fish oil might be a tolerable compromise and can elevate DHA content to 40 g/kg total FA. However, micro-algae may be a more acceptable means to elevate the DHA content of eggs, especially as its carotenoid content also improves yolk colour and acts as ‘natural’ antioxidant or preservative. Another approach may be to rely on consumption of legumes from range vegetation, which has been shown to elevate n-3 in milk and beef, although this is an area still to be investigated in poultry.
Milk and dairy produce
There has been considerable research carried out over the last 30 years or so, to understand how we can improve the fat composition of dairy products to be less harmful to our health. As with all ruminant products, butter is dominated by SFA despite relatively high PUFA intake from forage in many dairy diets. However, rumen microbes are also responsible for a unique group of FA( Reference Destaillats, Trottier and Galvez 17 ) (shown to benefit our health). These are hydrogenation intermediates that leave the rumen and become incorporated into milk and meat, before being fully converted to stearic acid (18 : 0). The most significant of these FA is an isomer of CLA, cis-9,trans-11-18 : 2 (CLA9), some of which is created from incomplete hydrogenation of LA and ALA in the rumen, although most is derived from the desaturation of VA (another intermediate of rumen hydrogenation) in the mammary gland or adipose tissue( Reference Griinari, Corl and Lacy 18 ).
Milk fat composition does vary and the most influential factor in causing this is known to be the diet consumed by cows especially their intake of fresh forage( Reference Dewhurst, Shingfield and Lee 5 ), although there is also a smaller genetic element, which potentially could be exploited by selective breeding. Considering that much of the variability between individual cows and herds is masked as milk is pooled and standardised throughout the supply chain, it is somewhat surprising that inconsistency in product quality exists for consumers. This is clearly illustrated in a simple study of retail milk, carried out over 2 years buying of organic and conventional milk in summer and winter( Reference Butler, Stergiadis and Seal 19 ). Results on the concentrations of PUFA are presented in Table 3 and can be summarised as: milk produced in summer, under organic management and/or if weather conditions are good is higher in beneficial FA compared with milk from winter, conventional management or if conditions are poor, respectively. Explanations for these differences might be found in a larger European study( Reference Butler, Nielsen and Larsen 20 ), which also highlights national variation in milk quality outwith the scope of the local retail study. Under a European research project investigating Quality and Safety in Low-Input Food milk quality was compared within Italy, Sweden, Denmark and the UK, collecting milk from farms under contrasting systems of management or geographical location, each represented by a cluster of commercial farms. Milk was collected on several occasions throughout the year along with detailed records of cow feeding and other management inputs. The published results( Reference Butler, Nielsen and Larsen 20 ) show elevated levels of CLA9 and ALA in milk tended to mirror forage intake, especially fresh grazing (see Fig. 2(a and b)) with levels in the UK milk substantially higher than many systems in other countries. Fresh growing forages are higher in ALA (Table 2) than other ingredients in dairy diets and elevated forage consumption raises ALA and linoleic acid intakes, some of which passes unchanged into the milk or is converted to VA, CLA9 and other isomers of CLA. Subsequent multivariate analysis of this European data (G Butler, unpublished results) shows the close association between predicted grazing intake and concentrations of ALA, n-3 and CLA9 (along with antioxidants α-tocopherol and lutein) in milk. Milk produced under organic or low-input management was significantly higher in ALA and total n-3 than other systems within each country; with the exception of one of the groups of organic farms in Italy (interestingly, their milk was higher in CLA9, compared with that from other systems). Milk produced by cows under organic or low-input management (diets dominated by grazing through most of the year) in the UK produced milk fat averaging over 11 g CLA9/kg total FA and almost 10 g ALA/kg total FA with samples from individual farms relying almost solely on grazing rather than supplementary feeding reaching 2·4 g CLA9 and 1·3 g ALA/kg total FA. This shows the potential to improve milk composition and it is interesting to note that even the average figures from this research are substantially higher than current recognised estimation of milk quality( Reference McCance and Widdowson 11 , 12 ).

Fig. 2. (a) Milk fat composition from European Farm survey; Concentrations of α-linolenic acid (ALA) and conjugated linoleic acid cis-9,trans-11-18 : 2 (CLA9) and (b) Diet composition: Breakdown of DM intake recorded on farms under different systems of production in different countries (average over all samples)( Reference Butler, Nielsen and Larsen 20 ). Mean values within countries were compared by Tukey's honest significant difference test, and those with the same letter do not differ significantly (P < 0·05) (a,b,c for α-linolenic acid and x,y,z for conjugated linoleic acid), ANOVA: *P < 0·05, **P < 0·01 and ***P < 0·001. Key to farm ID: Management: C = conventional, O = Organic LI = low input not organic. Italy: P = Potenza, C = Cosenza, B = Bologna, Mi = Milan and Mo = Modena. Sweden: HF = Holstein Friesian cows South, RS = Swedish Red cows South, RC = Swedish Red cows Central. Denmark: FM = frequent milking (>2 times/d), C = conserved forage feeding, S = standard, M = maize silage. UK: N = NE England, W = SW Wales.
Table 3. Variation in milk fat composition in NE England 2006–2008( Reference Butler, Stergiadis and Seal 19 )
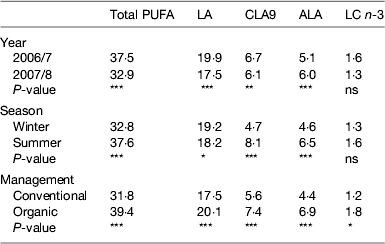
LA, linoleic acid cis-9,12-18 : 2, conjugated linoleic acid cis-9,trans-11-18 : 2 ALA, α-linolenic acid cis-9,12,15-18 : 3; LC n-3, long-chain n-3 fatty acids = EPA + docosapentaenoic acid +DHA.
*P≤0·05, **P≤0·01, ***P≤0·001.
While diets based solely on grazing are the ultimate in terms of ‘healthy’ milk, they are not necessarily a realistic option across the UK and in other regions; grass growth and/or grazing utilisation is restricted by extremes in precipitation and temperature. Unfortunately, not many parts of the UK can reliably grow sufficient forage to graze cows throughout the year and grass is preserved (usually fermented into silage) for winter feeding; hence the fluctuation in milk fat composition throughout the year as PUFA supply to cows is reduced on silage diets. In addition, modern dairy cows capable of high milk yields (current average of 7500 litres per lactation in the UK) need more concentrated feeds to support production and avoid metabolic diseases or impaired fertility.
A recognised substitute to replace PUFA in the absence of grazing is oilseeds; Glasser et al.( Reference Glasser, Ferlay and Chilliard 13 ) reviewed 145 trials assessing the effect of feeding linseed, rapeseed, soyabeans and sunflower seeds (or their extracted oils) on milk fat quality. As with the review on eggs, milk fat composition from the control diets in these studies was variable since almost half the FA concentrations reported were associated with a standard deviation >50 % of mean values. The FA contribution from these oilseeds is given in Table 2 and individual PUFA levels in milk differed significantly by adding them to dairy diets. Fig. 3 shows the average concentrations of the main PUFA groups in milk from cows fed with and without supplementation. Total CLA and CLA9 in milk were increased by all supplements (although differences were not significant in the case of rapeseeds or rape-oil) and LA was significantly higher in milk from cows receiving sunflower seeds or soyabeans (both relatively high in this n-6 fatty acid). These responses appear to be enhanced if the seeds or oils were protected from rumen modification, although a lack of detail presented in the present paper precludes inclusion of this data in Fig. 3. One slight concern in this respect is that although total n-3 content of milk is increased by linseed supplementation, this is not always associated with elevation in long-chain n-3 FA concentrations.

Fig. 3. Milk PUFA content from cows with and without dietary oilseed supplementation( Reference Glasser, Ferlay and Chilliard 13 ). CLA, conjugated linoleic acid.
Beef
When considering the nutritional attributes of red meat we tend to focus on protein quality, iron and other micronutrient content. Its important contribution to PUFA intake can be overlooked, since we generally regard beef fat being dominated by SFA, yet, lean beef can be a relevant source of n-3, especially in diets devoid of oily fish( Reference Howe, Meyer and Record 21 ). Although the overall PUFA content of red meat might be substantially lower than pork or poultry meat (taking the composition reported in Table 1), the n-3 contents are comparable and lean red meat had a superior n-6 : n-3 ratio often exceeding the dietary target of 1:1. As with milk, the fat profile of red meat is strongly influenced by production systems, giving scope to improve the supply of nutritionally beneficial PUFA.
There are a number of lipid depots in cattle and sheep and their composition tends to reflect their evolutionary/physiological function; some of which can be altered by dietary intervention, although to a lesser extent and less predictably than in non-ruminant livestock such as poultry. In trying to manipulate the fat profiles to benefit consumer health( Reference Scollan, Hocquette and Nuernberg 4 ), the two fat depots of greatest interest are intramuscular fat that generally is consumed within the meat and cannot be avoided and subcutaneous fat which, given preferences, can be trimmed prior to or after cooking. With regard to composition, the former can be considered as two distinct fractions: phospholipids forming the structural integrity of cell and organelle membranes and TAG deposited in adipose cells within the muscle, commonly referred to as marbling. The overall profile of fats within the muscle depends on the extent of the marbling and its composition, which is the fraction we can influence (by genetics, diet and overall fat levels within the carcass) since the phospholipid fraction is relatively consistent and reported to be high in PUFA (approximately 40 %( Reference Dannenberger, Nuernberg and Scollan 22 )).
Generally, subcutaneous fat is dominated by SFA and MUFA (especially palmitic acid; 16 : 0 and oleic acid; cis-9-18 : 1) with PUFA only comprising of 2–3 % of total FA( Reference Wood, Enser and Fisher 23 ). Intramuscular fat on the other hand is substantially higher in PUFA especially in lean beef where it can typically reach over 12 % of total FA( Reference Dannenberger, Nuernberg and Scollan 22 , Reference Kamihiro 24 ). The PUFA content of intramuscular fat and the relative proportions of CLA, n-3 and n-6 do fluctuate. Generally, lean beef has higher levels of n-3 and n-6 PUFA and these become diluted with SFA and MUFA as carcass fatness increases, particularly if fat is deposited within the muscle as marbling. In contrast to all other PUFA, CLA (along with its associated precursor VA) is higher in subcutaneous fat and marbling within muscle rather than lean tissue( Reference Kamihiro 24 ). Although some CLA9 is formed in the rumen, most is derived from VA desaturation by enzymes found in adipose tissue (and the mammary gland), hence higher concentration in adipose tissue rather than incorporation into phospholipids of cell membranes, like other PUFA. A small study of retail beef( Reference Kamihiro 24 ) shows a lean organic sirloin steak purchased in autumn could supply 158 mg ALA and 94 mg long-chain n-3 FA both of which meet over 33 % of recommended daily intakes. In addition, it will supply 223 mg CLA9 which could be increased substantially with consumption of visible subcutaneous fat. Irrespective of differences between subcutaneous fat and intramuscular fat, in practice, the profile of FA in beef is influenced directly and indirectly by many elements in its system of production; these include the breed, sex and age at slaughter of the animals as well as the types, quantity and quality of feeds used, with various interactions between these aspects. A simplified guide to maximising PUFA content of beef is given as Table 4.
Table 4. Simplified explanation of factors that influence PUFA content of intramuscular fat in beef mediated either (a) directly by dietary PUFA supply or (b) via the proportion of carcass fat (Note that interactions exist between the factors listed)( Reference Scollan, Hocquette and Nuernberg 4 , Reference Wood, Enser and Fisher 23 , Reference Kamihiro 24 )

CLA, conjugated linoleic acid.
Implications for health
Unfortunately, evidence to support the effect of these changes in the PUFA content of animal-derived foods on consumers’ health is scant, possibly due to the challenge of conducting controlled intervention studies for long enough to affect many of the chronic conditions associated with ageing such as cancer, CVD or type 2 diabetes. Although there is a lack of direct measurement of health there is a growing weight of evidence that altering the PUFA content of these foods does influence some recognisable indicators of health status, a few of which will be discussed.
Fraeye et al.( Reference Fraeye, Bruneel and Lemahieu 6 ) report several studies demonstrating that elevated n-3 are transferred from enriched eggs and perform bioactive roles within consumers, although this was not consistent across all the studies reviewed. A number report relatively rapid reduction (from 7 d) of serum TAG content, which is recognised as a predictor of CHD and there are also reported increases in DHA concentrations in platelet phospholipids (which may reduce platelet aggregation). This might be expected in situations where fish or algal supplementation of hens’ diets raises the DHA content of eggs but it was also reported in two papers where linseed supplementation gave only a marginal increase in DHA content of eggs. In these cases, a relatively large increase in egg ALA content appeared to be effective at raising DHA appearance in consumers.
Another study to support PUFA manipulation of food having a bearing on health is a large social study, KOALA Birth Cohort Study, conducted in the Netherlands to evaluate the role of organic food consumption on human health. Kummeling et al.( Reference Kummeling, Thijs and Huber 25 ) report that household consumption of organic dairy products was associated with lower risk of eczema in young children (OR 0·64 with 95 % CI 0–44, 0–93), possibly linked to their findings that nursing mothers consuming organic diets, produced milk higher in CLA.
As with the studies showing changes in plasma composition from eating enriched eggs, similar work has been reported by McAfee et al.( Reference McAfee, McSorley and Cuskelly 26 ) comparing red meat consumption from contrasting feeding regimes in Northern Ireland. For 4 weeks, consumers replaced their usual moderate red meat consumption with beef and lamb reared, either on a grazing system or cereal feeding during the last 6 weeks prior to slaughter. As expected, FA profiles in the meat differed between feeding systems with grass-fed livestock producing meat significantly higher in ALA, EPA, long-chain n-3 FA and total n-3 while being lower in LA and n-6 content. The lamb from grass feeding was also higher in CLA9 and docosapentaenoic acid compared with that finished on concentrated feeds. Fig. 4 presents the n-3 profiles in plasma and platelets FA of the study groups both before and after interventions on meat consumption. Despite a lack of significant difference in the DHA content of meat (or no traceable DHA in the case of beef mince), individuals consuming grass-fed meat had significantly higher levels of DHA in plasma and platelets, compared with those eating cereal-fed meat. This finding may also apply to elevated ALA content in milk following linseed supplementation and the effectiveness of this strategy for indirectly raising consumers’ DHA supply warrants further investigation.

Fig. 4. n-3 Fatty acid content of (a) plasma and (b) platelets in study groups following 4-week consumption of red meat from grass or cereal finishing systems( Reference McAfee, McSorley and Cuskelly 26 ). ALA, α-linolenic acid, DPA, docosapentaenoic acid. *P < 0·05, **P < 0·01: level of significance between the study groups pre- or post-intervention.
Conclusions
We are slowly gaining a better understanding of how we can manipulate the FA content of animal-derived foods to reduce the proportion of SFA and increase total and n-3 (long-chain) PUFA supply and there is growing evidence that this can have a positive effect on our health (indicators). However, as always, there is also a recognition that we may need to compromise between intensification and higher output at (relatively) reduced cost and this aspect of food quality.
Acknowledgements
I am grateful to colleagues for support, especially Carlo Leifert and Sokratis Stergiadis.
Financial Support
I gratefully acknowledge funding from the European Community under FP6-506358 QualityLowInputFood and FP7-222623 LowInputBreeds.
Conflicts of Interest
None.










