- HAPO
hyperglycaemia and adverse pregnancy outcome
Prevalence of obesity
The prevalence of obesity is rising worldwide. Scotland, in particular, has one of the highest prevalences of obesity of all Organisation for Economic Co-Operation and Development countries(1), with a prevalence of 22% among men and 24% among women in 2003. Given the above information, it is not surprising that a significant proportion of women enter pregnancy with a BMI which would define them as obese. Published data from Scotland suggest that 18·9% of women in Glasgow have a BMI of 30 or more at the time of pregnancy booking(Reference Kanagalingam, Forouhi and Greer2); these data concur with our own data in Lothian where 16·3% of women had a BMI of 30 or more at the time of booking in 2008(Reference Denison, Norrie and Graham3). Data from England suggest that the rise in rates of obesity in the general population is widely paralleled in the population of pregnant women(Reference Heslehurst, Ells and Simpson4, Reference Heslehurst, Rankin and Wilkinson5) and although prevalences of obesity in pregnant women remain 3–5% behind the general population, they are projected to be over 20% by the time of writing in 2011.
The consequences of obesity in non pregnant individuals have been extensively described. They include an increased risk of type 2 diabetes (typical fold increase of 12), hypertension (four-fold risk), myocardial infarction and colon cancer (each a three-fold risk) angina, gall bladder disease and ovarian cancer (two-fold risk) in addition to conditions whose incidence is only modestly increased by obesity such as stroke and osteoarthritis. A recent meta-analysis has shown that for every increase in BMI of 5 kg/m2 there is a 10% increase in neoplastic mortality, 40% increase in vascular mortality and a greater than 50% increase in diabetic, renal and hepatic mortality(Reference Whitlock, Lewington and Sherliker6). In contrast, the impact of obesity on reproductive outcomes has only recently been the subject of significant research(Reference Norman7). In pregnancy in the UK, attention has been drawn to this issue in the last two Confidential Enquiries on Maternal Mortality(8, 9). In the most recently reported triennium, 27% of women who died had a BMI of 30, a proportion in excess of the proportion of obese women in the maternity population. Thus, obese pregnant women appear to be at increased risk of death during pregnancy. Regarding specific causes of death, overweight or obese pregnant women were highly over-represented among those dying of thromboembolism (of whom 78% were overweight or obese) and cardiac causes (of whom 61% were overweight or obese)(8). Although disease pathophysiology clearly plays a role, the previous triennium reported that lack of basic equipment (e.g. lack of sphygmomanometer cuffs of appropriate size to measure blood pressure in obese pregnant women) and/or logistical issues (e.g. transport of obese pregnant women) could also be a factor in the death of some obese women(9).
In view of the increased mortality among obese pregnant women, the report of the 2002–2005 triennium recommended that ‘Preconception counselling and support, both opportunistic and planned, should be provided for women of childbearing age with pre-existing serious medical or mental health conditions which may be aggravated by pregnancy … . This includes obesity (BMI>30)(9).
While this recommendation is important and useful, it is less clear how pre-conception advice can improve outcome. Achieving a normal weight before embarking on pregnancy is a counsel of perfection; if weight cannot be lost the range of interventions which can improve outcome among obese women remains limited(10).
Antenatal pregnancy complications increased by obesity
Other than death, many adverse pregnancy outcomes are increased by obesity. They include maternal haemorrhage, maternal infection and longer hospital stay (Table 1)(Reference Heslehurst, Simpson and Ells11). In parallel with these major adverse outcomes, the ‘minor’ complications of pregnancy (heartburn, chest infection and symphysis pubis discomfort) are also increased in obese pregnant women, with evidence of a dose-dependent effect(Reference Denison, Norrie and Graham3). Although ‘minor’ in terms of risk of mortality, these events are not only distressing to the sufferer, but have an adverse economic impact. In extreme obesity (BMI>50) the risk of adverse outcomes increases further. The United Kingdom Obstetric Surveillance system study of extreme obesity identified that BMI>50 was associated with increased adjusted OR of a range of adverse outcomes, including gestational diabetes, thrombosis, pre-eclampsia, postpartum haemorrhage and intensive care unit admission (Table 2)(Reference Knight, Kurinczuk and Spark12).
Table 1. Adverse pregnancy outcomes increased by obesity (data from Heslehurst et al.(Reference Heslehurst, Simpson and Ells11))

Table 2. Adverse pregnancy outcomes increased by extreme obesity (data from Knight et al.(Reference Knight, Kurinczuk and Spark12))

Delivery complications
Delivery of an obese pregnant woman remains a challenge. Obese women are more likely to require both caesarean section and instrumental delivery(Reference Heslehurst, Simpson and Ells11) (Table 1), paradoxically obese pregnant women are at an increased risk of the complications associated with operative delivery. These risks are further exaggerated in morbid obesity(Reference Knight, Kurinczuk and Spark12) (Table 2), where operative delivery poses challenges to both the anaesthetist and obstetrician. Given the high likelihood of operative delivery, even where vaginal delivery is attempted, the fact that ‘emergency’ caesarean section carries greater risks than ‘elective’ caesarean section, and the need for experienced members of staff if operative delivery is needed, one could argue that women with morbid obesity might be best delivered by elective caesarean section. A prospective study of women with extreme obesity delivered by elective caesarean section compared with those in whom a vaginal delivery was planned showed no evidence of reduced complications in the caesarean section group, with the exception of a reduction in shoulder dystocia(Reference Homer, Kurinczuk and Spark13). As the authors note, these data do not support a policy of routine planned caesarean section for all morbidly obese women. In the Edinburgh metabolic antenatal clinic over the last few years, we have made a decision for delivery by elective caesarian on an individual patient basis after multidisciplinary discussion and careful consultation with the pregnant woman on a number of occasions.
Immediate effects of maternal obesity on the baby
The adverse effects of obesity in pregnancy are not confined to problems for the mother; the baby also is at risk from events arising in pregnancy. These events range from miscarriage and stillbirth, through fetal abnormality to macrosomia (Table 3)(Reference Heslehurst, Simpson and Ells11, Reference Chu, Kim and Lau14, Reference Metwally, Ong and Ledger15). A meta-analysis in the Lancet recently identified overweight and obesity as the single biggest modifiable factor in stillbirth in resource-rich countries(Reference Flenady, Koopmans and Middleton16). Additionally, the increased risk of a variety of maternal pregnancy complications results in an increased risk of induced preterm birth with a relative risk 1·30 (95% CI 1·23, 1·37)(Reference McDonald, Han and Mulla17).
Table 3. Adverse outcomes for the baby – data from Chu et al., Heslehurst et al. and Metwally et al. (Reference Heslehurst, Simpson and Ells11, Reference Chu, Kim and Lau14, Reference Metwally, Ong and Ledger15)

Weight gain during pregnancy
Given the known adverse effects of obesity in pregnancy, it is not surprising that these effects are mimicked or amplified by excess weight gain in pregnancy. Excess gestational weight gain is associated with increased mean birthweight and an increased incidence of babies being large for gestational age(Reference Siega-Riz, Viswanathan and Moos18). Conversely, inadequate gestational weight gain is associated with low birthweight and an increased incidence of babies being small for gestational age. For the mother, increasing evidence suggests that high gestational weight gain results in increased maternal obesity, as well as higher blood pressure, later in life(Reference Fraser, Tilling and Macdonald-Wallis19). Although there is clear evidence that ‘too much’ and ‘too little’ weight gain in pregnancy is not good, there are limited data to properly inform the ranges of appropriate weight gain. Notwithstanding, the Institute of Medicine in the USA recently issued guidelines on weight gain, suggesting for the first time that those with a high BMI at the onset of pregnancy should limit weight gain, compared with those with a normal or low BMI at booking(20). The Institute of Medicine recommendations are shown in Table 4. Importantly, there is as yet insufficient evidence to suggest that women with a very high BMI (BMI≥40) should lose weight in pregnancy; cohort studies suggest that although such a strategy can minimise the risk of macrosomia, caesarean section and pre-eclampsia, it does so at the expense of an increased incidence of babies that are small for gestational age(Reference Blomberg21).
Table 4. Institute of Medicine Recommendations for weight gain during pregnancy(20)

Programming of adverse pregnancy outcome
In addition to the immediate adverse consequences of maternal obesity and/or excess weight gain in pregnancy, growing evidence suggests that maternal obesity can ‘programme’ the baby for disease in future life(Reference Drake and Reynolds22). For example, Reichman and Nepomnyaschy showed that maternal obesity increases offspring asthma, with an OR of 1·52 (95% CI 1·18,1·93)(Reference Reichman and Nepomnyaschy23). Others have shown a possible link between maternal obesity and adverse neurodevelopmental outcomes, including cognitive problems and attention-deficit disorders in the baby(Reference Van Lieshout, Taylor and Boyle24). However, the most widely investigated programming effect of maternal obesity is on offspring obesity. There are now several observational studies supporting an association between maternal obesity and increased risk of obesity in the offspring as neonates, in childhood and in adolescence (see Drake and Reynolds for review(Reference Drake and Reynolds22)). We have recently shown that offspring fat mass and weight circumference in adulthood are also positively related to maternal BMI during pregnancy(Reference Reynolds, Osmond and Phillips25) independently of adult obesity lifestyle factors. In addition to these studies directly correlating offspring obesity in adulthood with maternal BMI, further studies using the surrogate of offspring birthweight support a link between maternal obesity and offspring obesity. As described earlier, maternal obesity is associated with high offspring birthweight. Linking birthweight and adult obesity, both the Nurses Health Studies (women) and the Health Professionals Follow-up study (men), large studies of about 163 000 and 22 000 sample size, respectively, showed a J-shaped association (in other words, a positive association at both ends of the curve) between birthweight and adult obesity(Reference Curhan, Chertow and Willett26, Reference Curhan, Willett and Rimm27). A systematic review by Parsons also demonstrated positive associations between birthweight and adult obesity, regardless of the method of obesity measurement (BMI, weight, skinfold thickness etc.)(Reference Parsons, Power and Logan28). This positive association between high birthweight (>4000 g) and adult obesity was confirmed again in the most recent systematic review with OR (95% CI) of adult obesity of 2·07 (1·91–2·24): interestingly this review suggested that the association between low birthweight and adult obesity disappeared after studies with selection bias were excluded(Reference Yu, Han and Zhu29).
The results of these epidemiological studies are recapitulated in animal studies, where maternal and offspring food intake can be tightly regulated, with environmental parameters carefully controlled between the groups. Bayol et al.(Reference Bayol, Simbi and Bertrand30) determined perirenal fat mass in rats exposed to a ‘junk food’ or a ‘normal’ diet, all on the background of either maternal junk food intake during pregnancy or normal diet during pregnancy. Not surprisingly, rats exposed to a junk-food diet both post-weaning and in utero had the greatest perirenal fat mass, and one that was substantially greater than rats never exposed to junk food. Rats exposed to junk food post-weaning, but not in utero, also displayed greater perirenal fat mass compared to normal-diet controls, albeit less pronounced than those exposed to junk food in utero. Of perhaps most surprise, however, rats exposed to junk food in utero, but then transferred to chow diet post-natally, also showed increased perirenal fat mass compared to controls (these differences reaching statistical significance in males but not females), indicating that the in utero effects of maternal obesity have consequences long beyond pregnancy. Although such studies cannot be replicated in human subjects, it appears likely that similar effects do occur although it is difficult to disentangle post-natal lifestyle influences. Catalano has postulated a scenario whereby maternal obesity during pregnancy begets fetal and neonatal obesity(Reference Catalano31). There is a potential for neonatal obesity to be modified in childhood by diet and exercise, but if it is not, it becomes childhood obesity (often accompanied by a decrease in insulin sensitivity), again with the potential modifiers of diet and exercise(Reference Lawlor, Benfield and Logue32), but if unmodified it becomes adult obesity (often accompanied by type 2 diabetes), which in women who become pregnant again exposes the fetus to obesity in utero and an abnormal metabolic environment(Reference Catalano31).
What mechanisms link obesity and adverse pregnancy outcome?
An understanding of mechanisms is required in order to devise therapies to treat the links between obesity and adverse pregnancy outcome. Several mechanisms have been postulated, but the ones backed by most evidence, and which will be discussed here, are hyperglycaemia and insulin resistance.
Impaired glucose tolerance
The links between diabetes and obesity are well recognised. The positive association between high glucose and high BMI remains, even below a ‘cut off’ level of glucose which would qualify for a diagnosis of diabetes. For example, Abbasi et al. showed a continuous positive association (r 0·465, P<0·01) between BMI and plasma glucose concentration(Reference Abbasi, Brown and Lamendola33), in a study of 300 healthy men. These results have been recapitulated in pregnancy, with maternal glucose concentrations of <99 mg/dl (5·5 mmol/l), 99–130 mg/dl and >130 mg/dl (7·2 mmol/l) in women in the Camden health study segregating women into those with a mean pre-gravid BMI of 23·2, 23·9 and 25·0, respectively (P<0·001)(Reference Scholl, Sowers and Chen34). This study also confirmed the veracity of the Pedersen hypothesis, that is, high maternal blood glucose results in increased nutrient transfer to the fetus and hence increased fetal growth(Reference Pedersen35), in that blood glucose concentrations also correlated with increasing risk of having a large-for-gestational-age baby: taking glucose of <99 mg/dl (5·5 mmol/l) as a referent, those with a medium (99–130 mg/dl) and high (>130) blood glucose had odds of a large-for-gestational-age baby of 1·4 and 3·59, respectively. Although Pedersen defined his hypothesis to explain macrosomia among babies of diabetic women, a similar paradigm appears to operate in women with a modestly elevated blood glucose but below the threshold for diagnosis of gestational diabetes.
In the Camden study, blood glucose segregated with rates of caesarean section as well as macrosomia. With glucose of <99 mg/dl as referent, medium and highest blood glucose levels had odds of caesarean section of 1·31 and 2·64, respectively.
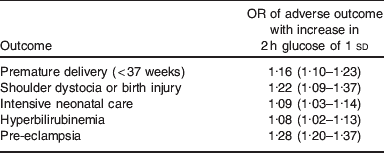
Although the Camden health study is large, with data on over 1000 women, it is dwarfed by the hyperglycaemia and adverse pregnancy outcome (HAPO) study which shows similar effects(Reference Metzger, Lowe and Dyer36). In HAPO, over 25 000 women underwent a 75 g glucose tolerance test in the late second or early third gestation. Those with gestational diabetes (fasting plasma glucose above 5·8 mmol/l of 2-h plasma glucose above 11·1 mmol/l) were identified and treated appropriately. The results of the glucose tolerance test remained concealed in the 23 000 women who did not have gestational diabetes. At the end of the study, the OR of adverse pregnancy outcomes were correlated against blood glucose profile. The prespecified primary outcomes were: birthweight above the 90th centile for gestational age, primary caesarean delivery, clinically diagnosed neonatal hypoglycaemia and cord-blood C-peptide level above the 90th centile. HAPO showed a direct relationship between each of birth weight above the 90th centile and primary caesarean section frequency, and blood glucose, with those in the highest blood glucose band having a greater proportion of women with babies >90th centile in weight and being delivered by primary caesarean section. There was also an increase in adverse outcomes of premature delivery, shoulder dystocia, requirement for neonatal intensive care, hyperbilirubinaemia and pre-eclampsia (Table 5). Thus, HAPO confirms the link between high blood glucose levels (even those not normally considered to qualify for gestational diabetes) and adverse pregnancy outcome, including high birthweight. Other studies have shown that this positive correlation between maternal blood glucose and offspring BMI may persist into early childhood(Reference Deierlein, Siega-Riz and Chantala37) although the association may not be apparent in very young children(Reference Pettitt, McKenna and McLaughlin38). That association between maternal glucose and adverse pregnancy outcome including high birthweight is causal (i.e. that high blood glucose causes adverse pregnancy outcome) rather than a mere association is evidenced by the Australian Carbohydrate Intolerance Study in pregnant women study, in which women with modestly impaired glucose tolerance (fasting glucose <7·8 mmol/l and 2 h post 75 g oral glucose load of 7·8–11·0 mmol) were randomised routine care or to diet and insulin(Reference Crowther, Hiller and Moss39). Although the risk of the composite adverse perinatal outcome was low in both groups 4 v. 1%, respectively, those randomised to diet and insulin had a lower incidence of the adverse outcome compared with those in the routine care group (relative risk 0·33, 95% CI 0·14, 0·75). Thus, there is good evidence that not only is modestly elevated blood glucose causal in the aetiology of adverse pregnancy outcome, but also that treatment (with diet and insulin) can ameliorate this causal link. Given that obese pregnant women have modestly elevated blood glucose compared to their lean counterparts, and have increased incidence of the adverse pregnancy outcomes (larger babies and caesarean section) compared to lean pregnant women, it is likely that elevated blood glucose concentrations mediate (at least in part) the effects of obesity on adverse pregnancy outcome.
Table 5. Secondary outcomes from the HAPO study(Reference Metzger, Lowe and Dyer36)
Longer term, there is emerging evidence that maternal blood glucose concentration programmes the offspring for later-life obesity. Dabelea's seminal study showing a greater incidence of obesity and diabetes in Pima Indians born after their mothers developed gestational diabetes, compared to their siblings born beforehand but no difference in the incidence of either diabetes or obesity after paternal development of diabetes(Reference Dabelea, Hanson and Lindsay40) provides important evidence of the programming effect of being exposed to high glucose levels in utero. This study has recently been confirmed in a study of 280 000 Swedish men, where the mean BMI of men aged 18 was 0·94 kg/m2 (95% CI 0·35, 1·52) greater in those who were born after compared to their siblings born before their mothers developed gestational diabetes, after adjustment for a variety of confounding factors(Reference Lawlor, Lichtenstein and Langstrom41).
Insulin resistance
The evidence for elevated maternal glucose mediating adverse pregnancy outcome is strong. In contrast, there is less evidence for the links between insulin resistance and adverse pregnancy outcome. However, obese pregnant women are insulin resistant compared to their lean pregnant counterparts(Reference Challier, Basu and Bintein42, Reference Ramsay, Ferrell and Crawford43). Challier et al. assessed insulin sensitivity from fasting glucose and insulin measurements using the Homeostasis Model Assessment and found mean Homoeostasis Model Assessment to be 4·3 (sd 0·5) in a group of obese pregnant women, compared with 1·2 (sd 0·3) in lean pregnant women (P<0·001) (i.e. increased insulin resistance in the obese). Ramsay et al. showed median (interquartile range) insulin levels to be 14·2 (11·3–27) and 6·15 (4·47–9·5) in obese v. lean pregnant women (P<0·0001), respectively, again suggesting increased insulin resistance in obese. To our knowledge, no studies have attempted to correlate insulin resistance with adverse pregnancy outcome. However, insulin resistance is a common finding in women with polycystic ovary syndrome (defined as two out of the three symptoms of oligo- or amenorrhoea, excess androgen activity and polycystic ovaries demonstrable on ultrasound examination): a meta-analysis of outcomes in pregnant women with a history of polycystic ovary syndrome showed an increased incidence of a variety of adverse pregnancy outcome including macrosomia (OR 1·13, 95% CI 0·73, 1·75) and caesarean section (OR 1·56, 95% 1·20, 2·02)(Reference Boomsma, Eijkemans and Hughes44) (Table 6). Thus, there is circumstantial evidence that insulin resistance may also link obesity to adverse pregnancy outcome.
Table 6. Adverse pregnancy outcomes in women with polycystic ovary syndrome(Reference Boomsma, Eijkemans and Hughes44)

NICU, neonatal intensive care unit.
Interventions
Understanding the links between obesity and adverse pregnancy outcome can inform effective therapeutic interventions. In non-pregnant individuals, diet and exercise are advocated to improve health. In pregnancy, there is limited evidence for the efficacy of diet and exercise(Reference Denison and Chiswick45) although several trials are underway to test these interventions (e.g. UPBEAT ISRCTN: 89971375).
Given the known links between obesity, elevated blood glucose, insulin resistance and adverse pregnancy outcome, we believe that metformin may be an appropriate therapy for obese pregnant women. Metformin is a biguanide which increases glucose uptake in the liver and skeletal muscle, and decreases hepatic glucose production, likely via increased AMP-activated protein kinase(Reference Zhou, Myers and Li46). Its use is endorsed as the first-line treatment for gestational diabetes(47)based on the results of the metformin v. insulin for the treatment of gestational diabetes study(Reference Rowan, Hague and Gao48). In the metformin v. insulin for the treatment of gestational diabetes study, 751 women with gestational diabetes, diagnosed between 20 and 33 weeks gestation, were randomised either to metformin, with or without insulin if necessary, or to insulin directly. The composite primary outcome was neonatal hypoglycaemia or respiratory distress or need for phototherapy or birth trauma or 5 min Apgar score less than 7 or prematurity. The incidence of the primary outcome was similar in both groups (32·0% in the metformin group and 32·2% in the insulin group), relative risk 0·99, 95% CI 0·80,1·23. There was no difference in birthweight. Just under 50% of the metformin group needed to take insulin in addition to metformin, but the remainder did not. Maternal weight gain (from enrolment to 36 weeks gestation) was, however, lower in the metformin group: 0·4 (sd 2·9) kg v. 2·0 (sd 3·3) kg, P<0·001.
We hypothesise that metformin, in reducing modestly elevated blood glucose and improving insulin resistance, might reduce adverse outcome in obese pregnant women. We are testing this hypothesis in the EMPOWaR study (ISRCTN 1279843), funded by the Medical Research Council, and managed by the Efficacy and Mechanisms Evaluation board on behalf of the National Institute for Health Research. In EMPOWaR, 400 obese pregnant women will be randomised to metformin or placebo from 12 to 16 weeks of pregnancy until delivery. The primary outcome is birthweight centile, but we will also look at the effect of metformin on vascular function, glucose disposal and baby fat mass. We hope that metformin may reduce mean birthweight (i.e. reduce excess birthweight centile) without increasing the risk of intrauterine growth retardation.
Summary
The ideal management of maternal obesity is prevention, but it seems unlikely that the increasing prevalence of obesity in pregnancy is going to change soon. The links between maternal obesity and adverse outcome are strong, and operate across a range of pregnancy complications. Further research is urgently needed to understand these links, in order to be able to develop therapies, and improve pregnancy outcome in this increasingly common condition.
Acknowledgements
J.E.N. and R.R. have funding from the charity Tommy's and from the Efficacy and Mechanism Evaluation (EME) programme (which is funded by the Medical Research Council (MRC) and managed by the National Institute for Health Research (NIHR)) for their research into obesity and pregnancy. The views expressed in this publication are those of the author(s) and not necessarily those of the MRC, NHS, NIHR or the Department of Health. Neither of the authors has any other conflict of interest in relation to this work. J.E.N. produced the first draft of the paper. Both J.E.N. and R.R. critically revised the manuscript and take responsibility for the final version.








