Seaweeds are a relatively unexploited source of antioxidants and there is potential for seaweeds to be utilised as health promoting ingredients in the pharmaceutical and functional food industries. Previous data from our laboratory demonstrated considerable antioxidant effects in certain seaweeds(Reference O'Sullivan, O'Callaghan and O'Grady1). The objective of the present study was to determine the effect of methanolic extracts of the seaweeds, Ascophyllum nodosum, Laminaria hyperborea, Pelvetia canaliculata, Fucus vesiculosus and Fucus serratus on the antioxidant status and DNA integrity of Caco-2 cells. Caco-2 cells were supplemented with increasing concentrations of the seaweed extracts for 24 h and cell viability was determined by the MTT assay. A concentration of 100 μg/ml was selected for all subsequent experiments. The effect of the extracts on the antioxidant status of the cells was assessed by measuring reduced glutathione (GSH) content following a 24 h exposure to the seaweed extracts(Reference Hissin and Hilf2). The activity of the antioxidant enzyme superoxide dismutase (SOD) was assessed following a 30 min exposure to 200 μm hydrogen peroxide (H2O2) which was preceded by a 24 h exposure to the seaweed extracts. DNA damage was also assessed using the comet assay(Reference Aherne and O'Brien3) in Caco-2 cells, which were pre-treated with each seaweed extract for 24 h followed by exposure to 50 μm H2O2 for 30 min.
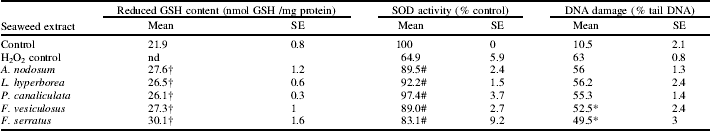
Data represent the mean of four individual experiments.
† Denotes significant difference (P<0.05) in GSH content, relative to untreated cells. # Denotes significant difference (P<0.05) in SOD activity, relative to H2O2 treated cells. * Denotes significant difference (P<0.05) in DNA damage, relative to H2O2 treated cells. Statistical analysis was by repeated measures ANOVA, followed by the Dunnett's test.
The seaweed extracts had no significant effect (P<0.05) on cell viability at concentrations below 2 mg/ml (data not shown). All extracts significantly (P<0.05) increased the GSH content in Caco-2 cells. F. serratus exhibited the greatest GSH enhancing effect, increasing the GSH levels by approximately 8 nmol GSHmg protein. The pre-incubation of Caco-2 cells with seaweed extracts helped to protect against the H2O2-mediated reduction of SOD activity. The presence of P. canaliculata provided almost complete protection against H2O2-induced SOD depletion. The addition of 50 μm H2O2 increased tail DNA to 63%, in Caco-2 cells. It was observed that pre-incubation with F. serratus and F. vesiculosus extracts significantly (P<0.05) reduced DNA damage. In conclusion, P. canaliculata, F. vesiculosus and F. serratus displayed the greatest antioxidant effect in the Caco-2 cell line as determined by the methods used in the present study. These seaweed extracts should be investigated further to assess their potential suitability for use in the functional food and pharmaceutical industry.
The Marine Functional Foods Research Initiative (NutraMara project) is a programme for marine-based functional food development established by the Marine Institute and the Department of Agriculture, Fisheries and Food (DAFF). It is supported by funds provided under the Strategy for Science, Technology and Innovation 2006–2013 (SSTI) and the Food Institutional Research Measure (FIRM), to establish a Marine Functional Foods Research Programme.



