- GPS
Glasgow prognostic score
Cancer is the leading cause of death worldwide among individuals aged 35–64 years and globally is responsible for >0·5×106 deaths annually. In the UK approximately one in three contract the disease in their life time and one in four of the population die from cancer(1).
Although much research is devoted to finding a cure for cancer, for the majority of patients with cancer the disease will progress either locally or become metastatic. Thus, anticipated survival is a major factor to be taken into consideration when deciding whether active intervention or palliation is appropriate.
Establishing the tumour stage of the patient has assumed paramount importance in the treatment of cancer. However, it is becoming increasingly recognised that the information that tumour stage provides on disease progression is inadequate. In particular, it is well recognised that predicting life expectancy of patients with advanced cancer is difficult and clinicians often overestimate survival(Reference Christakis and Lamont2, Reference Steensma and Loprinzi3). Current methods of assessing the suitability of such patients for treatment are usually based on host factors such as weight loss or performance status, since cancer patients who lose weight and have reduced performance status have a poorer prognosis than those who remain weight stable independent of tumour stage and anticancer therapies such as chemotherapy or radiotherapy treatment(Reference DeWys, Begg and Lavin4–Reference Andreyev, Norman, Oates and Cunningham6).
The clear link between weight loss, poor performance status and poor prognosis(Reference O'Gorman, McMillan and McArdle7–Reference O'Gorman, McMillan and McArdle9) is probably a result of the preferential loss of skeletal muscle(Reference Moley, Aamodt, Rumble, Kaye and Norton10–Reference McMillan, Preston, Watson, Simpson, Fearon, Shenkin, Burns and McArdle12). It has been suggested that the loss of adipose tissue accounts for the majority of the weight loss, but the loss of muscle accounts for most of the morbidity and mortality(Reference Nelson, Walsh and Sheehan13, Reference Toomey, Redmond and Bouchier-Hayes14).
However, the extent of weight loss that is prognostic is not well defined(Reference Morley, Thomas and Wilson15, Reference Fearon, Voss and Hustead16) and performance status is recognised to be subjective(Reference Ando, Ando, Hasegawa, Shimokata, Minami, Wakai, Ohno and Sakai17) and therefore their reliability has been questioned.
Aetiology of weight loss and reduced performance status
It is of interest that although the majority of patients with advanced cancer lose weight and have a poorer performance status, the extent varies according to tumour type. Patients with lung and gastrointestinal cancers tend to lose considerable amounts of weight and have reduced performance status early on in their illness.
Given that weight loss and reduced performance status are such an important problem in patients with cancer in terms of morbidity and mortality, the reversal of this process would seem to be a priority. However, the definitive method of treating these symptoms, i.e. removal of the tumour, is not an option in the majority of patients.
Weight loss results from an energy imbalance between energy intake and energy expenditure. This negative energy balance may, thus, be related to a reduced food intake, increased energy expenditure or a combination of both.
There have been many studies that have investigated the energy expenditure in patients with lung and gastrointestinal cancers(Reference Bozzetti18–Reference Falconer, Fearon, Plester, Ross and Carter21). Most studies have found an increased energy expenditure in patients with cancer who are losing weight, in contrast with the body's normal response to starvation that produces a reduced metabolic rate.
It has been reported that an elevated resting energy expenditure in patients with pancreatic cancer is associated with the presence of a systemic inflammatory response, as evidenced by an elevated C-reactive protein concentration(Reference Falconer, Fearon, Plester, Ross and Carter21). Similar results have been reported in other tumour types associated with weight loss, including lung cancer(Reference Staal-van den Brekel, Dentener, Schols, Buurman and Wouters22, Reference Scott, McMillan, Watson, Milroy and McArdle23).
It is therefore of interest that the presence of a systemic inflammatory response has also been shown to be associated with a reduction in the body cell mass (lean tissue) as measured by total body K(Reference McMillan, Preston, Watson, Simpson, Fearon, Shenkin, Burns and McArdle12, Reference McMillan, Scott, Watson, Preston, Milroy and McArdle24).
Further evidence of the central importance of the systemic inflammatory response is that the use of anti-inflammatory agents is associated with moderation of weight loss and the maintenance of performance status and quality of life in patients with advanced cancer(Reference Lundholm, Gelin and Hyltander25–Reference Lundholm, Daneryd, Körner, Hyltander and Bosaeus27).
Development of a systemic-inflammation-based score
There are a myriad of systemic responses to inflammation in human subjects resulting from infection, tissue injury, immunological disorders or cancer. These responses involve alterations in neuroendocrine metabolism (including the endocrine hormones), haematopoietic changes (including the IL, interferons and the haematopoietic growth factors), changes in protein and energy metabolism (including loss of muscle protein) and acute-phase proteins(Reference Gabay and Kushner28). The liver is central to the elaboration of the systemic inflammatory response. Hepatocytes are stimulated to synthesise and release into the systemic circulation a variety of acute-phase proteins, such as C-reactive protein, which initiate or sustain the systemic inflammatory response.
The systemic inflammatory response, manifested by elevation of C-reactive protein, may simply reflect a non-specific inflammatory response secondary to tumour hypoxia and necrosis or local tissue damage because apoptosis is a relatively ‘clean’ form of cell death in that it does not elicit an inflammatory immune response. These features are distinct from cells undergoing necrosis as a result of acute cell damage or ‘accidental’ cell death(Reference Thompson29).
In patients with cancer there is evidence of the stereotypical acute-phase protein response of C-reactive protein increasing and albumin falling, and this relationship is similar across different tumour types(Reference McMillan, Elahi, Sattar, Angerson, Johnstone and McArdle30) (Fig. 1). C-reactive protein, because of its sensitivity, specificity and reproducibility of analysis in hospital laboratories, is most commonly used to assess the magnitude (whether acute or chronic) of the systemic inflammatory response(Reference Gabay and Kushner28, Reference Vermeire, Van Assche and Rutgeerts31). Indeed, the magnitude of the increase in C-reactive protein concentrations has been shown to be associated with poorer survival in patients with cancer, particularly in patients with advanced disease(Reference Falconer, Fearon, Ross, Elton, Wigmore, Garden and Carter32–Reference Maltoni, Caraceni and Brunelli35).

Fig. 1. Relationship between C-reactive protein and albumin in a variety of common solid tumours. ( · · · ·), Colo-rectal cancer; (——), gastric cancer or breast cancer; (— · —), bronchogenic cancer. (From McMillan et al. (Reference McMillan, Elahi, Sattar, Angerson, Johnstone and McArdle30).)
It has been shown that in patients diagnosed with inoperable non-small-cell lung cancer and followed to death (n 106) there is, with increasing C-reactive protein concentrations from normal (<10 mg/l) to elevated (11–100 mg/l) and to highly-elevated (>100 mg/l), an increasing proportion of patients with >5% weight loss, poorer performance status and more fatigue(Reference Scott, McMillan, Forrest, Brown, McArdle and Milroy36). Also, the more elevated the C-reactive protein concentration the lower the albumin concentrations and the poorer the cancer-specific survival, independent of tumour stage.
In order to examine how the prognostic value of an elevated C-reactive protein concentration (>10 mg/l) might be used clinically, the prognostic value of the combinations of C-reactive protein and stage, C-reactive protein and performance status (Eastern Cooperative Oncology Group performance status, which assesses the well-being of patients with cancer and their ability to perform ordinary tasks(Reference Oken, Creech, Tormey, Horton, Davis, McFadden and Carbone37)), C-reactive protein and albumin (<35 g/l) together with stage and performance status were compared in 161 patients with inoperable non-small-cell lung cancer(Reference Forrest, McMillan, McArdle, Angerson and Dunlop38) (Table 1). On multivariate analysis, when the three scores based on the combinations of the systemic inflammatory response and stage, performance status and albumin were compared with the combination of stage and performance status, only the score based on the combination of the systemic inflammatory response and albumin (HR 1·70 (95% CI 1·23, 2·35); P<0·001) and the score based on stage and performance status (HR 1·48 (95% CI 1·12, 1·95); P=0·006) were found to retain independent significance.
Table 1. Cumulative prognostic scores and survival in patients with inoperable non-small-cell lung cancer (n 161); univariate survival analysis (from Forrest et al. (Reference Forrest, McMillan, McArdle, Angerson and Dunlop38))
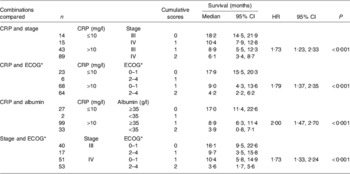
CRP, C-reactive protein; HR, hazard ratio.
* Eastern Cooperative Oncology Group performance status, which assesses the well-being of patients with cancer and their ability to perform ordinary tasks(Reference Oken, Creech, Tormey, Horton, Davis, McFadden and Carbone37).
The combination of C-reactive protein and albumin into a score (0, 1, 2) has much to commend it, since it has independent prognostic value, is simple to measure, routinely available and well standardised. This score now termed the Glasgow prognostic score (GPS) has been defined as follows: patients with both an elevated C-reactive protein (>10 mg/l) and hypoalbuminaemia (<35 g/l) are allocated a score of 2; patients in whom only one of these biochemical abnormalities is present are allocated a score of 1; patients in whom neither of these abnormalities is present are allocated a score of 0. However, in the clinical study the score of 1 was most commonly found to be a result of an elevated C-reactive protein (thirty-three of thirty-five patients), emphasising the inflammatory basis of the GPS(Reference Forrest, McMillan, McArdle, Angerson and Dunlop38).
Application of a systemic-inflammation-based score in patients with advanced cancer
The prognostic value of the GPS was then evaluated further in a variety of advanced cancers including non-small-cell lung(Reference Forrest, McMillan, McArdle, Angerson and Dunlop39), breast(Reference Al Murri, Bartlett, Canney, Doughty, Wilson and McMillan40), gastro-oesophageal(Reference Crumley, McMillan, McKernan, McDonald and Stuart41, Reference Crumley, Stuart, McKernan, McDonald and McMillan42), pancreatic(Reference Glen, Jamieson, McMillan, Carter, Imrie and McKay43), renal(Reference Ramsey, Lamb, Aitchison, Graham and McMillan44) and colo-rectal(Reference Leitch, Chakrabarti, Crozier, McKee, Anderson, Horgan and McMillan45) cancers. These studies (Table 2) have demonstrated that the prognostic value of the GPS is independent of tumour stage (all studies) and conventional scoring systems(Reference Ramsey, Lamb, Aitchison, Graham and McMillan44), superior to performance status(Reference Forrest, McMillan, McArdle, Angerson and Dunlop39, Reference Crumley, Stuart, McKernan, McDonald and McMillan42) and superior to other markers of the systemic inflammatory response such as leucocyte or lymphocyte counts(Reference Forrest, McMillan, McArdle, Angerson and Dunlop39, Reference Al Murri, Bartlett, Canney, Doughty, Wilson and McMillan40, Reference Crumley, Stuart, McKernan, McDonald and McMillan42, Reference Ramsey, Lamb, Aitchison, Graham and McMillan44, Reference Leitch, Chakrabarti, Crozier, McKee, Anderson, Horgan and McMillan45).
Table 2. Systemic inflammatory response, as evidenced by the Glasgow prognostic score (GPS), as a prognostic factor in advanced inoperable cancer

WCC, leucocyte count.
* Eastern Cooperative Oncology Group performance status, which assesses the well-being of patients with cancer and their ability to perform ordinary tasks(Reference Oken, Creech, Tormey, Horton, Davis, McFadden and Carbone37).
Having established a scoring system (the GPS) based on the systemic inflammation-driven loss of weight, lean tissue and performance status, it was of interest to examine its relationship with the general biochemical disturbance of patients with advanced cancer(Reference Brown, Milroy, Preston and McMillan46). The GPS was found to be normal in all the controls (n 13), but abnormal in 78% of the group with lung and gastrointestinal cancer (n 50). In addition to lower BMI and poorer performance status, serum concentrations of Na, chloride, creatine kinase, Zn and vitamin D were found to be lower in the group with cancer, whereas concentrations of Ca, Cu, alkaline phosphatase and γ-glutamyl transferase were raised. In the patient group, with increasing GPS a median reduction was found in Karnofsky performance status(Reference Karnofsky, Burchenal and MacLeod47) (25%), Hb (22%), Na(3%), Zn (15%) and survival (93%) and a median increase in leucocyte count (129%), alkaline phosphatase (217%), γ-glutamyl transferase (371%) and lactate dehydrogenase (130%). C-reactive protein concentrations were found to be strongly and similarly correlated with alkaline phosphatase and γ-glutamyl transferase, accounting for >25% of the variation in their activities. Thus, it would appear that chronic activation of the systemic inflammatory response in cancer is associated with important aspects of the general biochemical disturbance in patients with advanced cancer(Reference Brown, Milroy, Preston and McMillan46).
Thus, it is concluded that the combination of an elevated C-reactive protein concentration and hypoalbuminaemia (the GPS) is a tumour stage and performance status independent prognostic factor in patients with advanced inoperable cancer.
Application of a systemic-inflammation-based score in patients with primary cancer
There has also been some work in primary operable cancer that has shown that the systemic inflammatory response, as evidenced by an elevated C-reactive protein concentration, has prognostic value in gastro-oesophageal(Reference Ikeda, Natsugoe, Ueno, Baba and Aikou48, Reference Crumley, McMillan, McKernan, Going, Shearer and Stuart49), urinary bladder(Reference Hilmy, Bartlett, Underwood and McMillan50), pancreatic(Reference Jamieson, Glen, McMillan, McKay, Foulis, Carter and Imrie51), renal(Reference Lamb, McMillan, Ramsey and Aitchison52, Reference Karakiewicz, Hutterer, Trinh, Jeldres, Perrotte, Gallina, Tostain and Patard53) and non-small-cell lung(Reference Hara, Matsuzaki, Shimuzu, Tomita, Ayabe, Enomoto and Onitsuka54) cancers, independent of tumour stage (Table 3). Also, a number of studies carried out in primary operable colo-rectal cancer have highlighted the independent prognostic value of an elevated C-reactive protein concentration(Reference McMillan, Wotherspoon, Fearon, Sturgeon, Cooke and McArdle55–Reference McMillan, Crozier, Canna, Angerson and McArdle62), with only two studies failing to observe such a relationship(Reference Wigmore, McMahon, Sturgeon and Fearon63, Reference Chung and Chang64).
Table 3. Systemic inflammatory response, as evidenced by C-reactive protein (CRP), as a prognostic factor in primary operable cancer

Pre-op, pre-operative; post-op, post-operative; GPS, Glasgow prognostic score.
Recently, the prognostic value of the GPS has been examined in patients with either primary operable colo-rectal cancer (n 149) or synchronous unresectable liver metastases (n 84)(Reference Leitch, Chakrabarti, Crozier, McKee, Anderson, Horgan and McMillan65). The GPS was found to be a superior predictor of cancer-specific survival compared with leucocyte components of the systemic inflammatory response.
Thus, it is concluded that markers of the systemic inflammatory response, in particular C-reactive protein, are independently associated with survival in patients with primary operable cancer. The combination of an elevated C-reactive protein concentration and hypoalbuminaemia (the GPS) is a tumour stage- and treatment-independent prognostic factor in patients with primary operable colo-rectal cancer.
In summary, it is believed that a measure of the systemic inflammatory response, such as the GPS, should be included together with tumour stage as part of the assessment of the patient with cancer. As a consequence, this approach will highlight the need not only to treat the tumour but also the systemic inflammatory response.
Acknowledgements
The author declares no conflict of interest. The author gratefully acknowledges the support and advice of clinical and scientific colleagues at Glasgow Royal Infirmary and funding from Glasgow Royal Infirmary Endowment Funds, the Chief Scientist Office and Cancer Research UK.






