Article contents
Crystal structure of Ca2Zn2(V4O14) and Pb2Cd2(V3O10)(VO4) double vanadates
Published online by Cambridge University Press: 14 June 2018
Abstract
Polycrystalline samples of Ca2Zn2(V4O14) (I) and Pb2Cd2(V3O10)(VO4) (II) were synthesized using the nitrate–citrate method (I) and conventional solid state reaction (II). The structural refinement based on X-ray powder diffraction data showed that the crystal structure of (I) is characterized by monoclinic symmetry with unit-cell parameters a = 6.8044(1) Å, b = 14.4876(3) Å, c = 11.2367(2) Å, β = 99.647(1)° [space group P21/c (No. 14), Z = 4], and the crystal structure of (II) is triclinic with unit-cell parameters a = 7.03813(6) Å, b = 12.9085(1) Å, c = 6.99961(5) Å, α = 90.7265(5)°, β = 96.3789(5)°, γ = 94.9530(6)°, V = 629.470(8) Å3 [space group P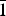 $\bar 1$ (No. 2), Z = 2].
$\bar 1$ (No. 2), Z = 2].
- Type
- New Diffraction Data
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2018
References
- 2
- Cited by



