Published online by Cambridge University Press: 19 September 2018
The crystal structures of Ca1.5Mn0.5V2O7 (I) and Ca1.5Cd0.5V2O7 (II) synthesized by the citrate method and by a conventional solid-state reaction, respectively, were determined using X-ray powder diffraction data. It was found that the compound I has a monoclinic crystal structure a = 4.88563(9) Å, b = 11.21279(22) Å, c = 5.69643(11 Å), β = 96.376(7)°, V = 310.132(10) Å3 (space group P21/c), Z = 2). Compound I has a narrow homogeneity region Ca1.5±0.1Mn0.5±0.1V2O7. The vanadate Ca1.5Cd0.5V2O7 crystallizes in the triclinic system with the parameters a = 6.66139(6) Å, b = 6.93019(7) Å, c = 7.02211(6) Å, α = 85.4404(9)°, β = 63.7505(7)°, γ = 82.5515(10)° и V = 288.201(5) Å3 (space group P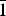 $\bar 1$, Z = 2). It is one of the formulations of the primary solid solution, formed as a result of the substitution of part of the calcium cations for cadmium cations in Ca2V2O7.
$\bar 1$, Z = 2). It is one of the formulations of the primary solid solution, formed as a result of the substitution of part of the calcium cations for cadmium cations in Ca2V2O7.