Introduction
Human trichomoniasis is one of the most common non-viral sexually transmitted diseases. It affects about 278 million people worldwide (WHO, 2012) and is caused by the parasite Trichomonas vaginalis. Trichomoniasis induces an infectious process in the urogenital tract, and in pregnant women, the disease can lead to severe complications: increased risk of placental membrane rupture, premature birth and chronic pulmonary disease acquired during childbirth (Hirt, Reference Hirt2013). In men, the disease is often asymptomatic, but it can cause urethritis, prostatitis and infertility (Secor, Reference Secor2012). T. vaginalis can cause infertility in both men and women (Lewis, Reference Lewis2010; Mielczarek and Blaszkowska, Reference Mielczarek and Blaszkowska2016). Trichomoniasis has received a great deal of attention due to the severe consequences of the disease, such as increased risks for HIV infection and cervical and prostate cancers (Secor, Reference Secor2012; Hirt, Reference Hirt2013).
The 5-Nitroimidazole compounds, such as metronidazole [1-(2-hydroxyethyl)-2- methyl-5-nitroimidazole] (Edwards, Reference Edwards1993), are recommended as trichomoniasis treatments. Although metronidazole has high efficacy, it has several negative side effects (Secor, Reference Secor2012). These include nausea, vomiting, metallic taste in the mouth, diarrhea and peripheral neuropathies (Rosa et al., Reference Rosa, Rocha, de Souza, Urbina and Benchimol2011). Moreover, several studies documented metronidazole resistance (Houang et al., Reference Houang, Ahmet and Lawrence1997; Schwebke and Barrientes, Reference Schwebke and Barrientes2006). Therefore, the development of a safer compound for the treatment of this infection is needed.
Clotrimazole (CTZ) has been used for Candida albicans infections and other diseases, such as sickle cell disease and some cancers (Crowley and Gallagher, Reference Crowley and Gallagher2014). In addition, clotrimazole was previously used for the treatment of metronidazole-resistant trichomoniasis (Cudmore et al., Reference Cudmore, Delgaty, Hayward-McClelland, Petrin and Garber2004). When clotrimazole is combined with other molecules, such as metals, it displays promising pharmacological characteristics (Navarro et al., Reference Navarro, Peña, Colmenares, González, Arsenak and Taylor2006, Reference Navarro, Higuera-Padilla, Arsenak and Taylor2009). Assuming that the zinc present in the prostatic fluid has microbicidal effects on T. vaginalis (Levy and Fair, Reference Levy and Fair1973; Benchimol et al., Reference Benchimol, Almeida, Lins, Gonçalves and de Souza1993), we investigated the activity of zinc-clotrimazole complexes against the parasite. Here, we demonstrate a significant anti-proliferative effect of the zinc-clotrimazole complexes in vitro against T. vaginalis.
Materials and methods
Parasite and cell culture
For the analyses of drug activity against T. vaginalis, we used the JT strain isolated in the Hospital of Federal University of Rio de Janeiro in Brazil. Trophozoites were maintained in Diamond's trypticase yeast-extract maltose (TYM) medium supplemented with 10% foetal bovine serum (FBS), and the cells were grown for 24 h at 37 °C. A human foreskin fibroblast (HFF; ATCC) cell line was chosen to evaluate the drug's cytotoxicity. HFFs were grown in 25-cm2 culture flasks containing Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 2 mm L-glutamine and 10% FBS at 37 °C under a 5% CO2 atmosphere.
Chemicals and instrumentation
All commercial reagents (CTZ, ZnCl2 and ZnAc2.2H2O) were used without further purification. Analytical-grade solvents were distilled from appropriate drying agents prior to use. The Infrared (IR) spectra were obtained using a Shimazu spectrometer in the 4000–400 cm−1 region. The elemental composition of the metal complexes was determined using a Perkin Elmer 2400 CHNS/O Series II microanalyzer. Conductivity values were obtained using a Meter Lab CDM2300 instrument. Nuclear Magnetic Resonance (NMR) experiments were performed at 298 K on a Bruker DRX 400 MHz spectrometer at 9.4 T, and 1H was observed at 400.13 MHz, while 13C{1H} was observed at 100.62 MHz. The NMR spectra were recorded in dimethyl sulfoxide (DMSO)-d6 solutions with tetramethylsilane TMS.
Synthesis of zinc-clotrimazole complexes
[Zn(CTZ)2(Ac)2].2H2O
To prepare [Zn(CTZ)2(Ac)2].2H2O, 710 mg (2.06 mm) of CTZ was dissolved in methanol (30 mL), and 205 mg (0.93 mm) of ZnAc2.2H2O was added to the solution, which was then stirred at room temperature for 48 h. The volume of the solvent was reduced, and distilled water was added. The white solid obtained was filtered, washed with cold methanol and dried under vacuum (yield: 778 mg, 94%). Elemental analysis: Calculated for C48H40N4Cl2O4Zn.2H2O: C, 63.41; H, 4.88; N, 6.16. Found: C, 63.41; H, 5.30; N, 6.05. IR (cm−1): ν(O-H) 3440, ν(C = N) 1585, ν(C = C) 1492, ν a(COO) 1618 and ν s(COO) 1433. ¹H NMR [DMSO; δ ppm (multiplicity, integration, assignation): 7.77 (s, 1H, H2); 7.51 (m, 2H, H9 and H8); 7.41 (m, 7H, H7, H10, H12, H14, H15, H17, H19); 7.11 (s, 1H, H5); 7.06 (m, 4H, H11, H13, H16, H18); 6.96 (s, 1H, H4); 6.91 (d, 1H, H6, J ortho = 7.84 Hz), 1.75 (s, 3H, Ac). Molar Conductivity in DMSO, ΛM = 0.35 ± 0.03 Ω−1 cm2 m−1.
[Zn(CTZ)2Cl2]
To prepare [Zn(CTZ)2Cl2], 308 mg (0.89 mm) of CTZ was dissolved in 20 mL of methanol, and a solution of 58 mg (0.43 mm) of ZnCl2 in 10 mL of methanol was added. The mixture was stirred at room temperature for 24 h. The white solid obtained was filtered, washed with distilled water and dried under a vacuum (yield, 291 mg, 82%). Elemental analysis: Calculated for C44H34N4Cl4Zn: C, 63.98; H, 4.15; N, 6.78. Found: C, 64.52; H, 3.53; N, 6.51. IR (cm−1): ν(C = N) 1589, ν(C = C) 1492. ¹H NMR [DMSO; δ ppm (multiplicity, integration, assignation): 7.67 (s, 1H, H2); 7.52 (m, 2H, H9 and H8); 7.42 (m, 7H, H7, H10, H12, H14, H15, H17, H19); 7.11 (s, 1H, H5); 7.07 (m, 4H, H11, H13, H16, H18); 7.03 (s, 1H, H4); 6.93 (d, 1H, H6, J ortho = 7.71 Hz). Molar Conductivity in DMSO, ΛM = 3.57 ± 0.05 Ω−1 cm2 m−1. The same compound was independently synthesized using a slightly different procedure (Betanzos-Lara et al., Reference Betanzos-Lara, Gómez-Ruiz, Barrón-Sosa, Gracia-Mora, Flores-Álamo and Barba-Behrens2012).
In vitro anti-proliferative activities
Parasites were grown to a density of 2 × 104 cells mL−1. Next, CTZ and the zinc-clotrimazole complexes, [Zn(CTZ)2(Ac)2] and [Zn(CTZ)2Cl2], were added at different concentrations (1, 5, 10 or 20 µ m) from stock solutions diluted in DMSO. The final concentration of DMSO in the growth medium never exceeded 1% (v/v), and it had no effect on cell growth or cell morphology. Cell densities were determined using a haemocytometer.
Combination treatment
We performed a combination treatment in order to evaluate the combined effect of CTZ with ZnAc2. The drugs were added as separated compounds following the ratio of 2:1 (CTZ: ZnAc2). In [Zn(CTZ)2(Ac)2], for each zinc atom there are two molecules of clotrimazole molecule in the complex. Based in the concentration of zinc-clotrimazole complexes we set the combination treatment equalizing both compounds with the same number of available molecules. For construction of the association growth curve, we treated the parasites simultaneously with 1:2, 5:10, 10:20 and 20:40 µ m of ZnAc2: Clotrimazole, respectively. Cell densities were determined using a haemocytometer.
Determination of IC50
To determine the drug concentration that inhibited parasite growth by 50% (IC50), the percentage of growth inhibition was plotted as a function of the drug concentration, and the values were fitted in a non-linear curve. Regression analyses were performed using the Sigma Plot 8.0 software. Data were plotted using the Graphpad Prism 6.0® software.
Immunofluorescence
Control or treated parasites were washed in warm PBS (pH 7.2) and allowed to adhere to glass coverslips with poly-L-lysine for 10 min at 37 °C. Cells were fixed for 1 h with 4% formaldehyde, permeabilized with 3% Nonidet (NP-40) and incubated with 50 mm NH4Cl and 3% of bovine serum albumin in PBS (PBS/BSA) as blocking solutions, after each step mentioned above the samples were washed with PBS (pH 8.0). Trichomonas were labeled with polyclonal antibody anti-pyruvate:ferrodoxin oxidoreductase (PFOR) (Provided by Dr Jan Tachezy, Charles University, Czech Republic). Afterwards, the coverslips were washed with PBS (pH 8.0) and incubated for 1 h with an Alexa Fluor 488-conjugated anti-mouse IgG antibody (Life Technologies) that was diluted 1:100 in PBS/BSA. Finally, the coverslips were washed and examined using an Axio Observer fluorescence microscope (Zeiss, Germany).
Scanning electron microscopy (S.E.M.)
Parasites were adhered as described above and fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2). They were then post-fixed for 15 min in 1% OsO4. The parasites were dehydrated in an ascending series of ethanol into 100% ethanol, critical-point dried with liquid CO2, coated with a 15-nm thick layer of gold and observed using a Quanta s.e.m. (FEI company, The Netherlands). For high-resolution observations, the sputter-coating step was performed using a thin layer (2 nm) of platinum, and cells were observed on an Auriga High-Resolution s.e.m. (Zeiss, Germany).
Transmission electron microscopy (TEM)
Control and treated trichomonas were fixed as described previously for s.e.m. After fixation, cells were post-fixed for 40 min in a solution containing 1% OsO4 and 0.8% potassium ferrocyanide in 0.1 M cacodylate buffer. They were then washed in PBS, dehydrated in acetone and embedded in Epon (an epoxy resin). Ultrathin sections (60–70-nm thick) were stained with uranyl acetate and lead citrate and were observed using a Tecnai Spirit TEM (FEI company, the Netherlands).
Viability analysis
To measure parasite viability, non-treated and IC50 concentration-treated T. vaginalis were stained with 6 µ m of propidium iodide (PI) (Sigma, USA) for 30 min. The analyses were performed after 48 h of incubation with the drug. Cell fluorescence was analyzed using an Accuri flow cytometer (BD Biosciences, USA). Overall, three independent experiments were performed in duplicate. The cytotoxic effects of the compounds on HFF cells and the viability of T. vaginalis when treated with all compounds were evaluated based on the reduction of MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4 sulfo-phenyl) 2H-tetrazolium] into the soluble formazan dye (Promega, USA). For this assay, 2 × 105 HFF cells were seeded into 96-well plates and cultured with DMEM supplemented with 2 mm L-glutamine and 10% FBS at a final volume of 200 µL per well. After the cells reached confluence, they were incubated for 48 h with clotrimazole and complexes (1) and (2) at different concentrations in DMEM supplemented with 2 mm L-glutamine and 10% FBS. The dilution concentrations ranged from 10–50 µ m for clotrimazole and 5–30 µ m for complexes 1 and 2. At the end of the experiment, the HFF cells were washed with PBS and incubated with 100 µL of 10 mm glucose in PBS plus 20 µL of MTS (Promega, USA). The same methodology was applied for T. vaginalis, where the initial amount of cells was 2 × 104 parasites per well. The absorbance was determined at 490 nm after 2 h of incubation in the microplate reader SpectraMax M2e (Molecular Devices). Cytotoxicity was calculated as a percentage of viable cells vs untreated cells (control, 100%). Control cells were treated with 4% formaldehyde.
Results
Synthesis and characterization
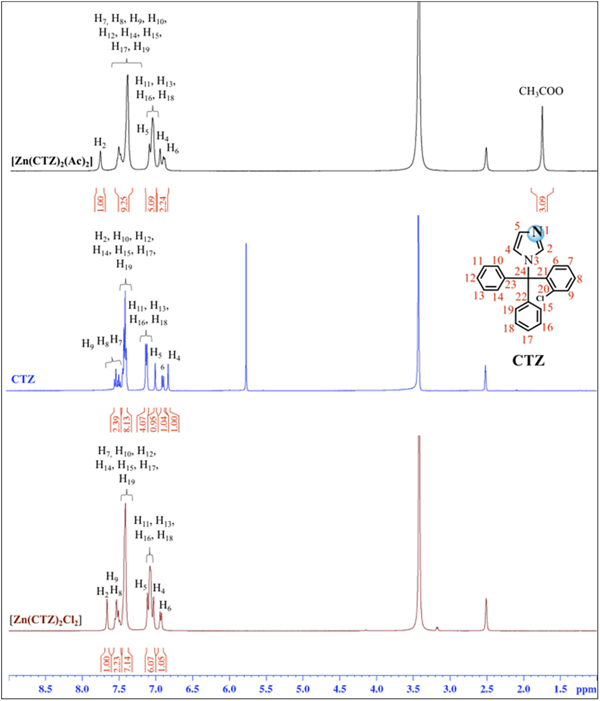
Figure 1 shows the synthesis scheme for [Zn(CTZ)2(Ac)2] [1] and [Zn(CTZ)2Cl2] [2], which were obtained at a high yield. All of these metal complexes were obtained as air-stable white solids. The elemental analyses determined were in agreement with the molecular formula proposed for each Zn-CTZ complex. In order to analyze if these metal complexes were neutral or cationic, their molar conductivity were measured in DMSO, finding that they are in the range for non-electrolyte complexes (17). The IR spectra of complexes 1 and 2 showed frequencies clearly associated with the characteristic functional group of the clotrimazole ligand, as well as the characteristic bands for each metal salts used in this synthesis. In the case of complex 1, signals that corresponded to the stretching vibrations could be observed due to the acetate ligand-binding unidentate form of the metal centre [which resulted from the differences between the values of ν a and ν s COO− (185 cm−1) that were greater than the free ligand (164 cm−1)]. Based on the 1H-NMR spectra results, it is possible to propose that CTZ bound to zinc by the unsubstituted N1 atom of the imidazole, due to the 1H chemical shift variation observed for the Zn-CTZ complexes with respect to those of the free CTZ (Δδ). A significant downfield shifts (Δδ) was observed for the imidazole protons attached to the zinc, as an example, H2 was 0.36 ppm for complex 1 and 0.25 ppm for complex 2 with respect to free CTZ (Fig. 2). It was also possible observed for complex 1, than the relative integral of the signals for the methyl protons of the acetate group in the spectrum was in agreement with an acetate group:CTZ ratio of 1:1.
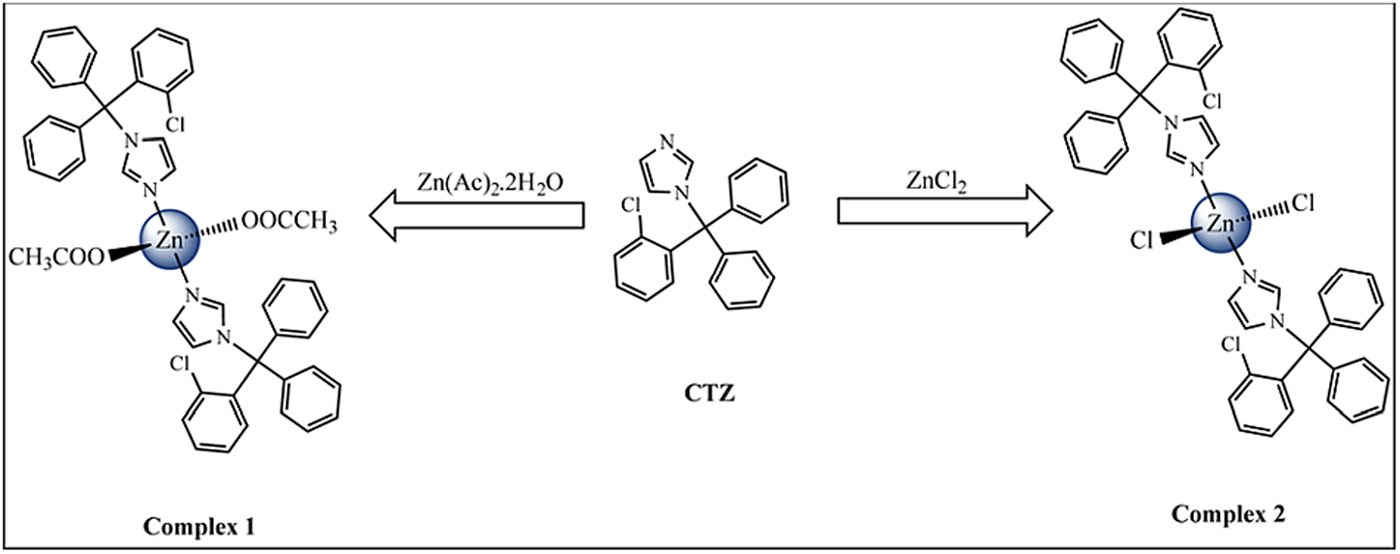
Fig. 1. Scheme of synthesis of Zn-CTZ complexes.

Fig. 2. 1H NMR spectra of complexes [Zn(CTZ)2(Ac)2], [Zn(CTZ)2Cl2] and CTZ free in DMSO-d6.
Anti-proliferative effects
When we added CTZ and its zinc complexes in different concentrations (1, 5, 10 and 20 µ m) to T. vaginalis cultures after 24 h of initial parasite growth, they exhibited a dose-dependent reduction in cell growth (Fig. 3, Supplementary Fig. 1). The IC50 (Supplementary Fig. 2) and IC90 values obtained after a 48 h incubation period for all compounds were as follows: CTZ, 17.2 µ m and >40 µ m (Fig. 3a); [Zn(CTZ)2Cl2], 10.5 and 22.9 µ m (Fig. 3c) and [Zn(CTZ)2(Ac)2], 4.9 and 17.2 µ m (Fig. 3d). In addition, it is important to highlight that at the final concentrations used, DMSO affected neither parasite growth (Fig. 3) nor morphology when observed by light, s.e.m. and TEM (data not shown). These results indicated that the [Zn(CTZ)2(Ac)2] compound was more effective against the parasite, it reduced IC50 values by around 70% (Fig. 3, Supplementary Fig. 1). To confirm the superior effect of the [Zn(CTZ)2(Ac)2] complex, we evaluated the combination of CTZ plus the salt ZnAc2 against T. vaginalis (Fig. 3e, Supplementary Fig. 1). For this experiment we used a ratio of 2:1 CTZ: ZnAc2, once in the Zn-CTZ complexes formula the CTZ is twice per mole. The concentrations used in this experiment were 1:2, 5:10, 10:20 and 20:40 µ m of ZnAc2: Clotrimazole, respectively. The IC50 value for CTZ added in combination with ZnAc2 after 48 h of treatment was 15.7 µ m (Fig. 3e, Supplementary Fig. 2), which very close to the IC50 value of CTZ alone (17.2 µ m). Thus, this result confirms that the [Zn(CTZ)2(Ac)2] compound (Fig. 3, Supplementary Fig. 1) is more effective against T. vaginalis than the CTZ, even when its used twice concentration.
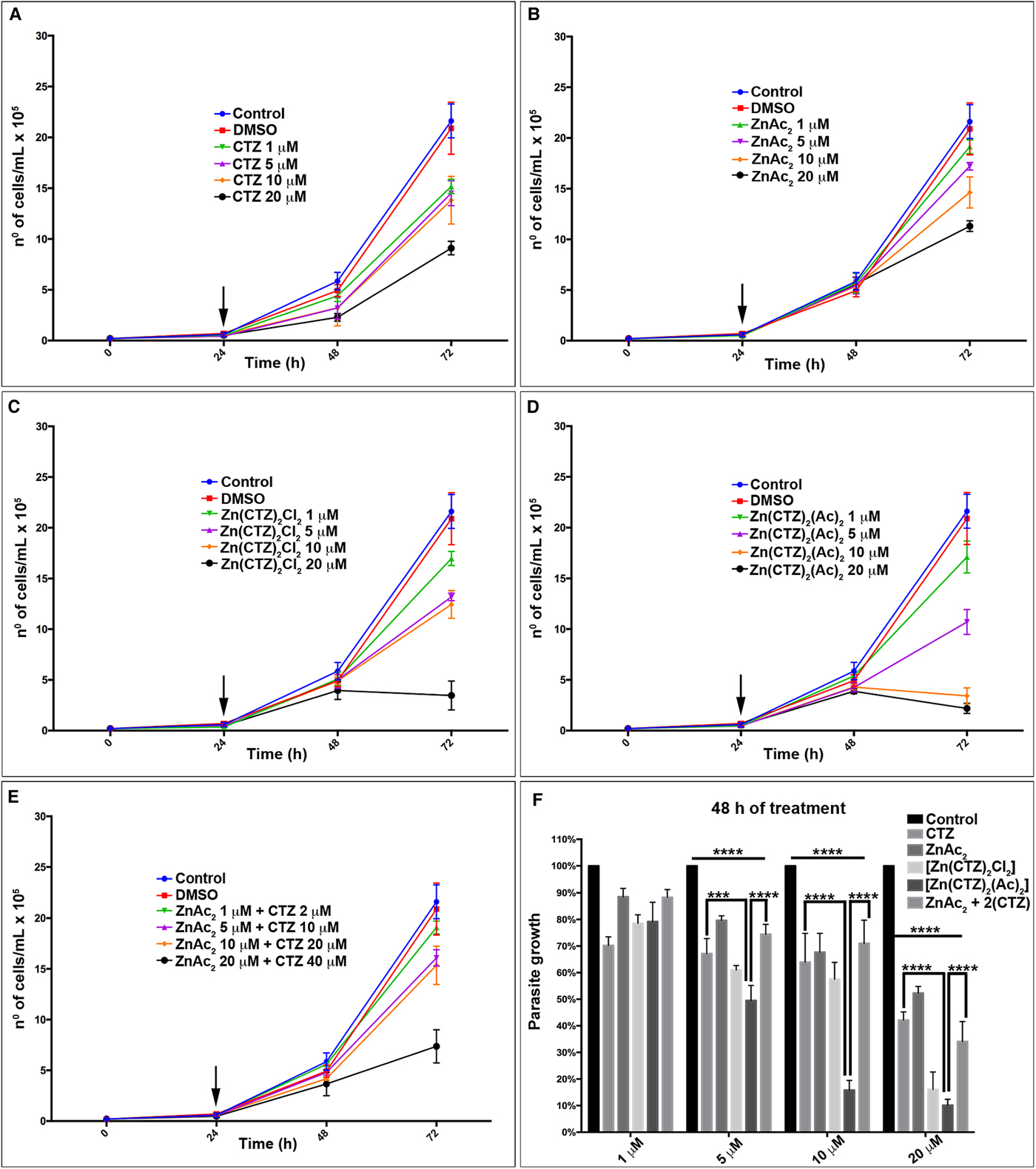
Fig. 3. Antiproliferative effect of clotrimazole and zinc -clotrimazole complexes against T. vaginalis. The growth curve of parasites treated with different concentrations of (a) non-complexed clotrimazole, CTZ; (b) zinc acetate salt, ZnAc2; and zinc –clotrimazole complexes (c) [Zn(CTZ)2Cl2] and (d) [Zn(CTZ)2(Ac)2]; (e) zinc acetate salt with non-complexed clotrimazole combination, ZnAc2 + CTZ. Combined treatment (e) were performed using the ratio of 2:1 (CTZ:ZnAc2). The compounds were added after 24 h (arrow) of cell growth. The number of cells was determined by light microscopy using a haemocytometer. (f) Percentage of parasite growth treated with different concentrations of non-complexed CTZ, ZnAc2, Zn-CTZ complexes: [Zn(CTZ)2Cl2] and [Zn(CTZ)2(Ac)2], and ZnAc2 + CTZ combination after 48 h of treatment. Note that in 48 h of treatment (f) there is a significant alteration comparing control with treated parasites, CTZ and ZnAc2 + CTZ combination with [Zn(CTZ)2(Ac)2] compound. Statistical significance of differences among the groups was assessed using the two-way analysis of variance (2way-ANOVA) test followed by Turkey's multiple comparison test in the GraphPad Prism 6 Software. Results were considered statistically significant when P ⩽ 0.03. (****P < 0.0001; ***P < 0.001). The data represent the mean ± Standard Deviation (s.d.) of three independent experiments performed in triplicate for each compound.
A comparison of parasite growth in percentage was performed (Fig. 3f, Supplementary Fig. 1e). We assumed in each time point the control as 100% and the percentage value of all compounds used was relative to its control. This data corroborates the growth curves (Fig. 3a–e); the [Zn(CTZ)2(Ac)2] compound presented the most significant effect when compared with CTZ treated parasites (P value <0.0001) and the ZnAc2 + CTZ combined treatment (P value <0.001) (Fig. 3f).
Ultrastructural effects
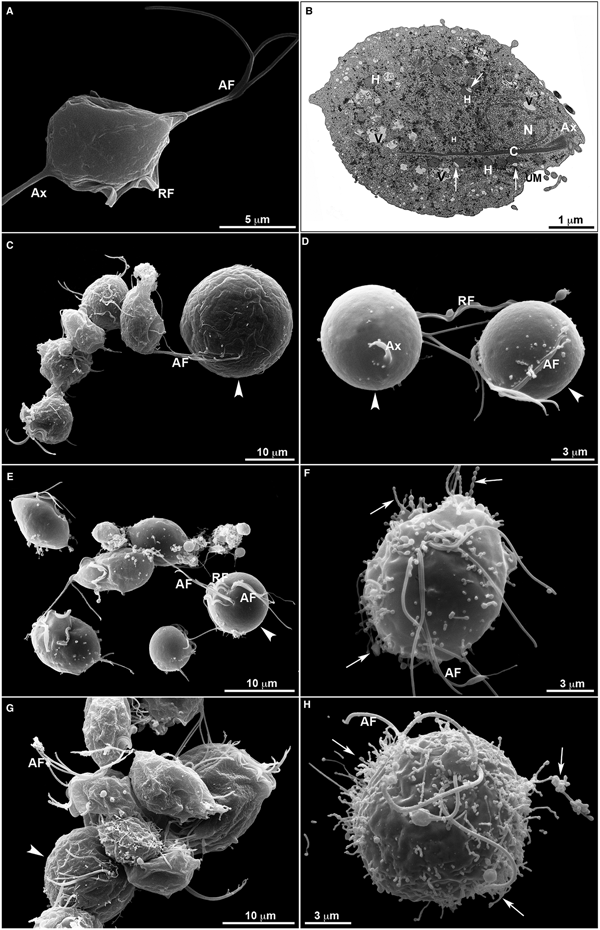
Untreated T. vaginalis (Fig. 4a–b) displayed a normal morphology when visualized both by s.e.m. (Fig. 4a) and TEM (Fig. 4b). By s.e.m., control cells showed a pear-shaped body, a protruding axostyle, an adhered recurrent flagellum and four anterior flagella emerging from the anterior region of the parasite's cell body (Fig. 4a). Moreover, using TEM, the nucleus of non-treated trichomonas was positioned at the anterior region, and hydrogenosomes, the membranous profiles of the endoplasmic reticulum, Golgi complex and vacuoles were observable (Fig. 4b). However, parasites incubated in the presence of the compounds displayed several ultrastructural alterations (Figs 4–7): rounded cells, the presence of surface projections, atypical hydrogenosomes and endoplasmic reticulum (Fig. 6).
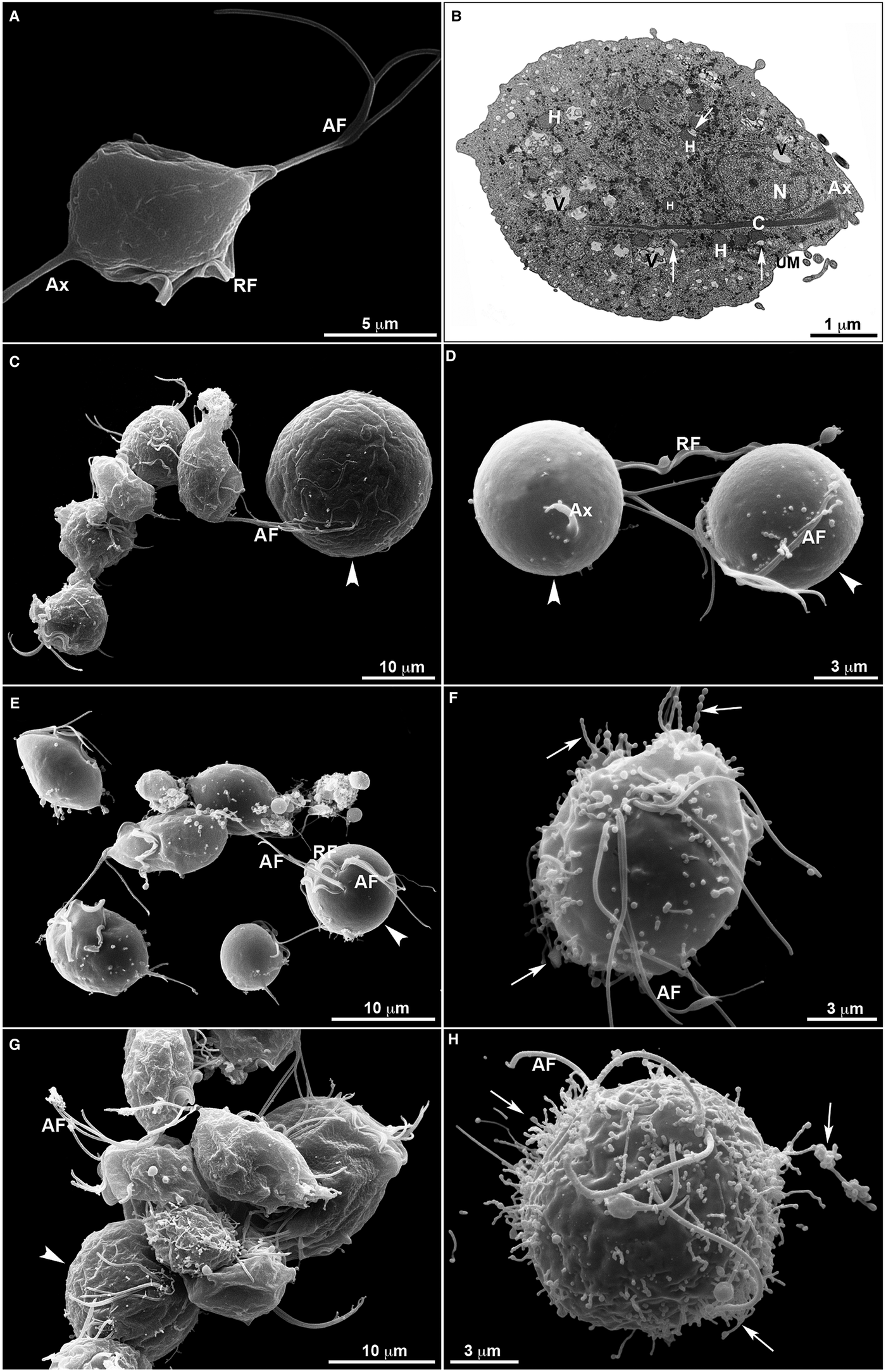
Fig. 4. Ultrastructural features of T. vaginalis by electron microscopy. (a) s.e.m. and (b) TEM of untreated parasites are seen. The cells exhibited regular morphology, including a pear shape, four anterior flagella (AF), a recurrent flagellum (RF) and an axostyle (Ax). (c–h) Morphological alterations in drug-treated parasites visualized by s.e.m. T. vaginalis were incubated with 19 µ m of clotrimazole (c–d), 10.3 µ m of [Zn(CTZ)2Cl2] (e–f) and 5.1 µ m of [Zn(CTZ)2(Ac)2] (g–h). All compounds altered the morphology of the parasites, including cell rounding (arrowhead) induced by both non-complexed (c–d) and zinc-complexed clotrimazole agents, [Zn(CTZ)2Cl2] (e–f) and [Zn(CTZ)2(Ac)2] (g). In rounded cells, the flagella are externalized (c–e, g). Membrane projections (filopodia) are visible (arrows) (f, h), which are enhanced with [Zn(CTZ)2(Ac)2] treatment (g–h). AF, anterior flagella; Ax, axostyle; C, costa; N, anteriorly positioned nucleus; H, hydrogenosomes with peripheral vesicle (arrows); RF, recurrent flagellum; UM, undulating membrane; V, vacuoles.
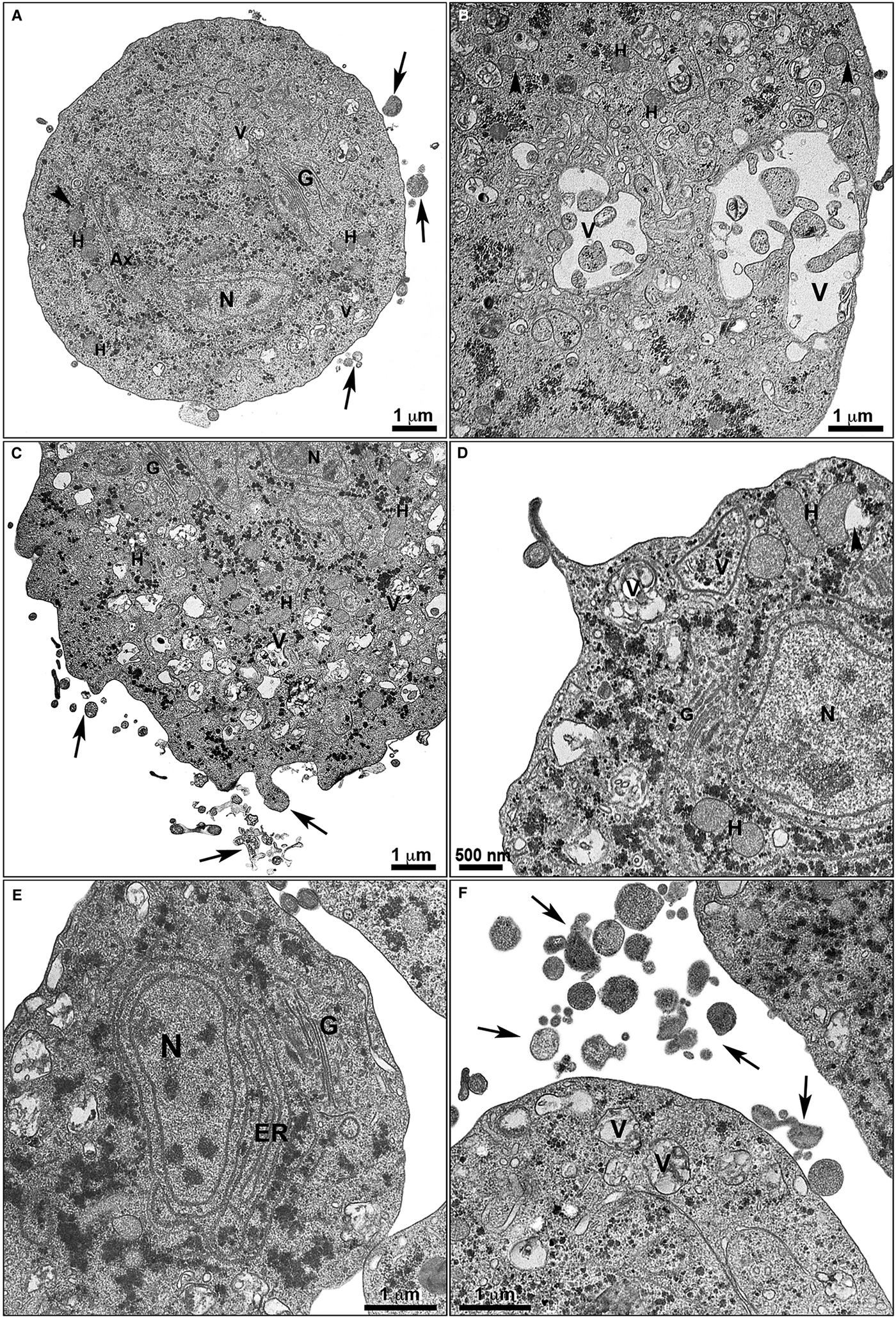
Fig. 5. Ultrastructural alterations in treated T. vaginalis. The cells treated with non-complexed (a-b) and zinc-complexed (c-f) clotrimazole are observed by TEM. Parasites treated with 19 µ m of clotrimazole (a–b) displays fewer membrane projections (arrows) when compared with the cells treated with 101 µ m of [Zn(CTZ)2Cl2] (c–d) and 5,1 µ m of [Zn(CTZ)2(Ac)2] (e–f), which exhibited a greater number of filopodia (arrows). An irregular distribution of Golgi lamellas (a, c, e) and abnormal hydrogenosomes (arrowhead) are observed (a–b, d). The ER is atypical and enlarged following [Zn(CTZ)2(Ac)2] treatment (e), and involves cytoplasmic structures (e). Ax, Axostyle; ER, endoplasmic reticulum; G, Golgi complex; H, hydrogenosome; N, nucleus; V, vacuoles.
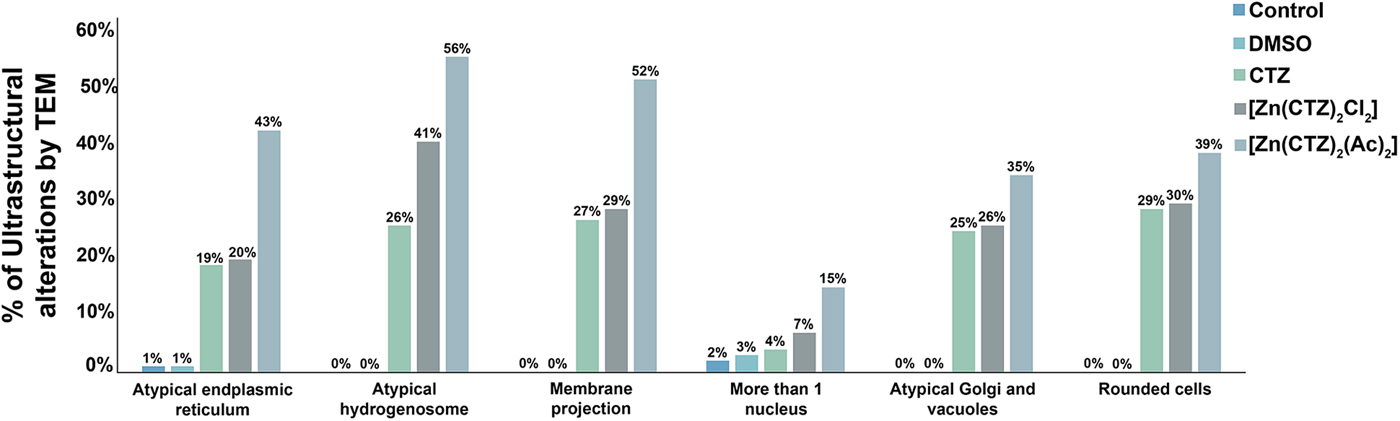
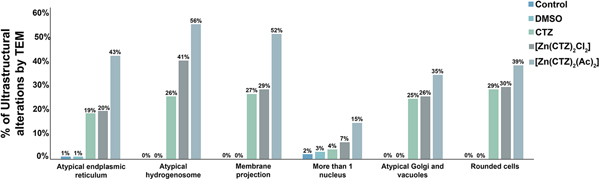
Fig. 6. Quantitative analysis of the effects of clotrimazole on parasite ultrastructure. One hundred parasites were counted using TEM. The values are expressed in percentages. Hydrogenosomes and the plasma membrane were primarily affected in both non-complexed and zinc-complexed clotrimazole. The [Zn(CTZ)2(Ac)2] compound induces atypical hydrogenosomes and membrane projections in approximately 56% and 52% of the treated parasites, respectively.
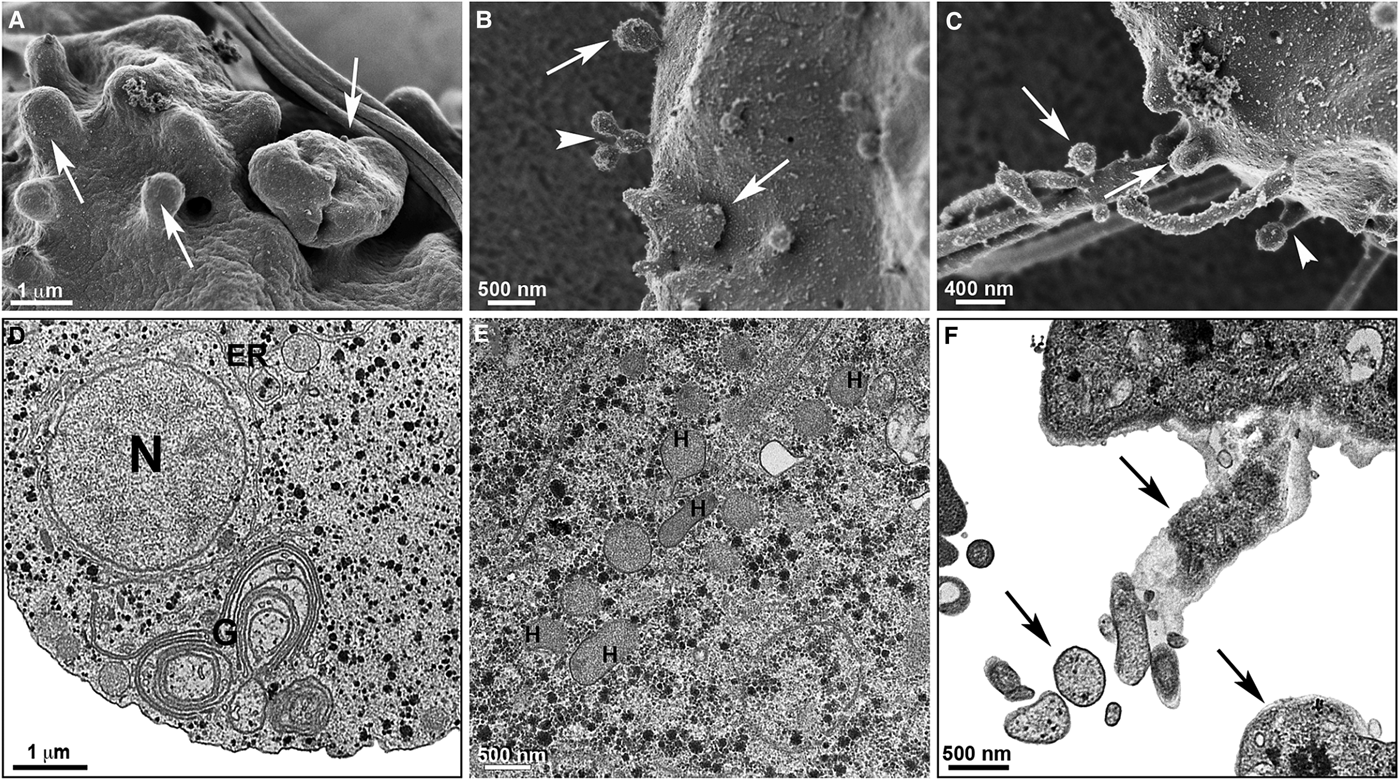
Fig. 7. Effects of [Zn(CTZ)2(Ac)2] treatment on T. vaginalis. Trophozoites were treated with 5.1 µ m of [Zn(CTZ)2(Ac)2] and observed by High resolution-scanning electron microscopy (HR-s.e.m.) (a–c) and TEM (d–f). Protrusions of plasma membrane are identified (arrows) by HR-s.e.m. (a–c). Membrane projections (arrowhead) welled up from the plasma membrane (b–c). Atypical Golgi complex (G) (d) and hydrogenosomes (H) are observed by TEM (e). Cytoplasmic components are seen in the membrane projections (arrows) (f). ER, endoplasmic reticulum; N, nucleus; V, vacuoles.
The extent of ultrastructural alterations found in 48 h-treated parasites and were different when CTZ, [Zn(CTZ)2Cl2] and [Zn(CTZ)2(Ac)2] were compared (Fig. 6). We observed many altered cells when zinc-CTZ complexes were used compared to non-complexed CTZ (Fig. 6). Furthermore, [Zn(CTZ)2(Ac)2] was the most effective compound, and quantitative analyses revealed that more than 50% of treated parasites exhibited non-organized membrane projections (Figs 4–7) and atypical hydrogenosomes (Figs 4–8). Although rounded cells were frequently observed following the use of all compounds (Figs 4–6), endoflagellar forms were not identified (Fig. 4a), and cells displayed externalized flagella (Fig. 4a–c and e). Membrane projections were only seen in treated cells (Figs 4, 5 and 7) as highlighted in Fig. 6. They corresponded to surface projections on the protozoan's plasma membrane filled with cytoplasmic content (Figs. 5c, 5f and 7f). When T. vaginalis was treated with [Zn(CTZ)2Cl2] and [Zn(CTZ)2(Ac)2], these surface extensions detached from the cell body (Figs 5f and 7f). Using high-resolution s.e.m., we observed some membrane projections that appeared as stretched structures welling up from the plasma membrane (Fig. 7a–c). Besides the membrane projections, other important alterations in [Zn(CTZ)2(Ac)2]-treated parasites were observed (Fig. 7d–e), including disorganized Golgi lamellae (Figs 5e and 7d) and altered hydrogenosomes (Fig. 7e).
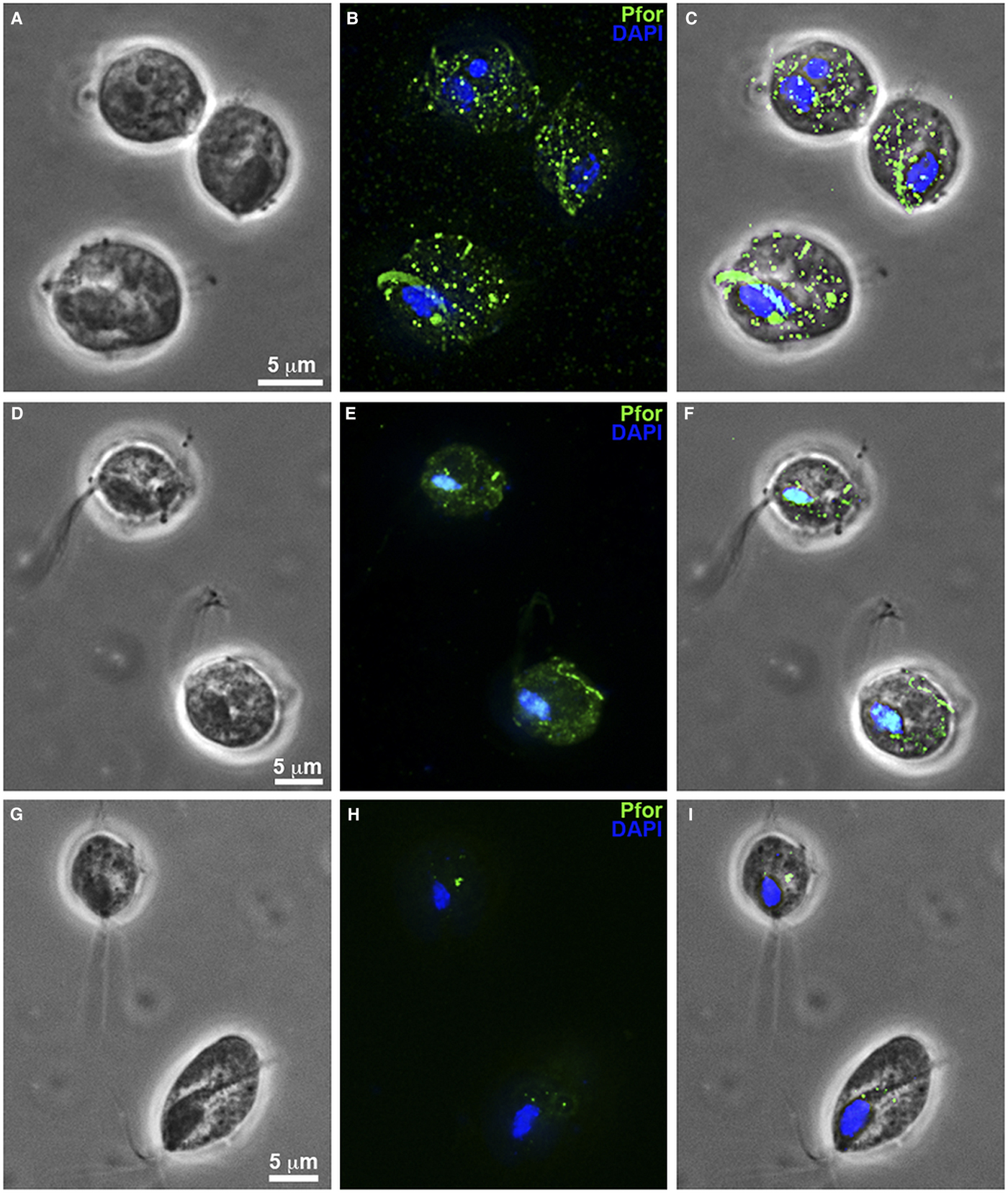
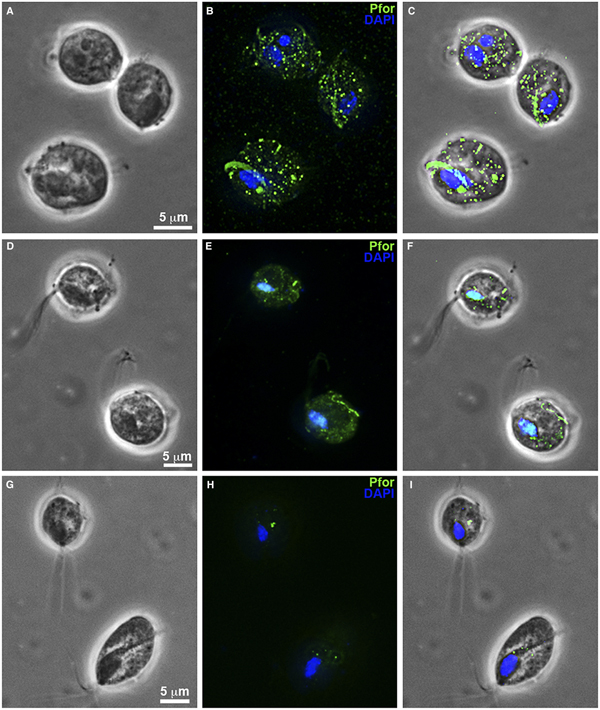
Fig. 8. T. vaginalis hydrogenosomes after treatment with clotrimazole compounds under fluorescence microscopy. (a–c): Non-treated parasites. (d-f): T. vaginalis treated with 19 µ m of non-complexed clotrimazole. (g–i): Parasites treated with 5.1 µ m of [Zn(CTZ)2(Ac)2]. (b, e, h): Hydrogenosomes labelled with the antibody anti-pyruvate:ferridoxin oxidoreductase (anti-PFOR). Treated cells (d–i) exhibited a reduction in fluorescence. T. vaginalis treated with [Zn(CTZ)2(Ac)2] (g–l) displays less signal than cells treated with non-complexed CTZ (d–f). (a, d, g): Differential interferential contrast (DIC). (c, f, I): overlay. Nuclei stained in blue with DAPI.
Hydrogenossomes
Hydrogenosomes are a good target for developing novel chemotherapies against T. vaginalis. Here, we observed alterations in organelle morphology (Figs 5d and 7e) and number, which was indicated by the visualization of hydrogenosomes by immunofluorescence microscopy after immunostaining cells with the anti-PFOR antibody (Fig. 8d–i) following incubation with the IC50 concentration of CTZ and its zinc complexes. It was possible to observe hydrogenosomes with no visible peripheral vesicles (Figs 5a–d and 7e) and no spherical shape (Figs 5c–d and 7e). Immunofluorescence microscopy (Fig. 8) showed a striking difference in hydrogenosome distribution when the parasites were treated with either CTZ (Fig. 8d–f) or [Zn(CTZ)2(Ac)2] (Fig. 8g–i). Non-treated cells displayed a regular distribution of hydrogenosomes and immunolabelling (Fig. 8a–c).
Viability assay
Quantitative viability assays were performed using PI for parasites (Fig. 9a), MTS for human cells (Fig. 9b) and T. vaginalis (Supplementary Fig. 3). The administration of CTZ and zinc complexes against T. vaginalis revealed that, for all compounds, there was no observable change in cell permeability due to membrane loss in the remaining protozoa when compared with controls and DMSO-treated cells (Fig. 9a). The plasma membrane integrity of parasites treated with these compounds for 48 h was similar to that of untreated cells. After 72 h of culture growth, 94.5% of untreated parasites were viable (Fig. 9a) as determined using PI. At 48 h following drug incubation, 96% of CTZ-treated cells, 95% of [Zn(CTZ)2Cl2]-treated cells and 96% of [Zn(CTZ)2(Ac)2]-treated cells were viable (Fig. 9a). The same profile was observed with MTS assay (Supplementary Fig. 3), there were no significant differences in the viability percentages among these treatment groups.
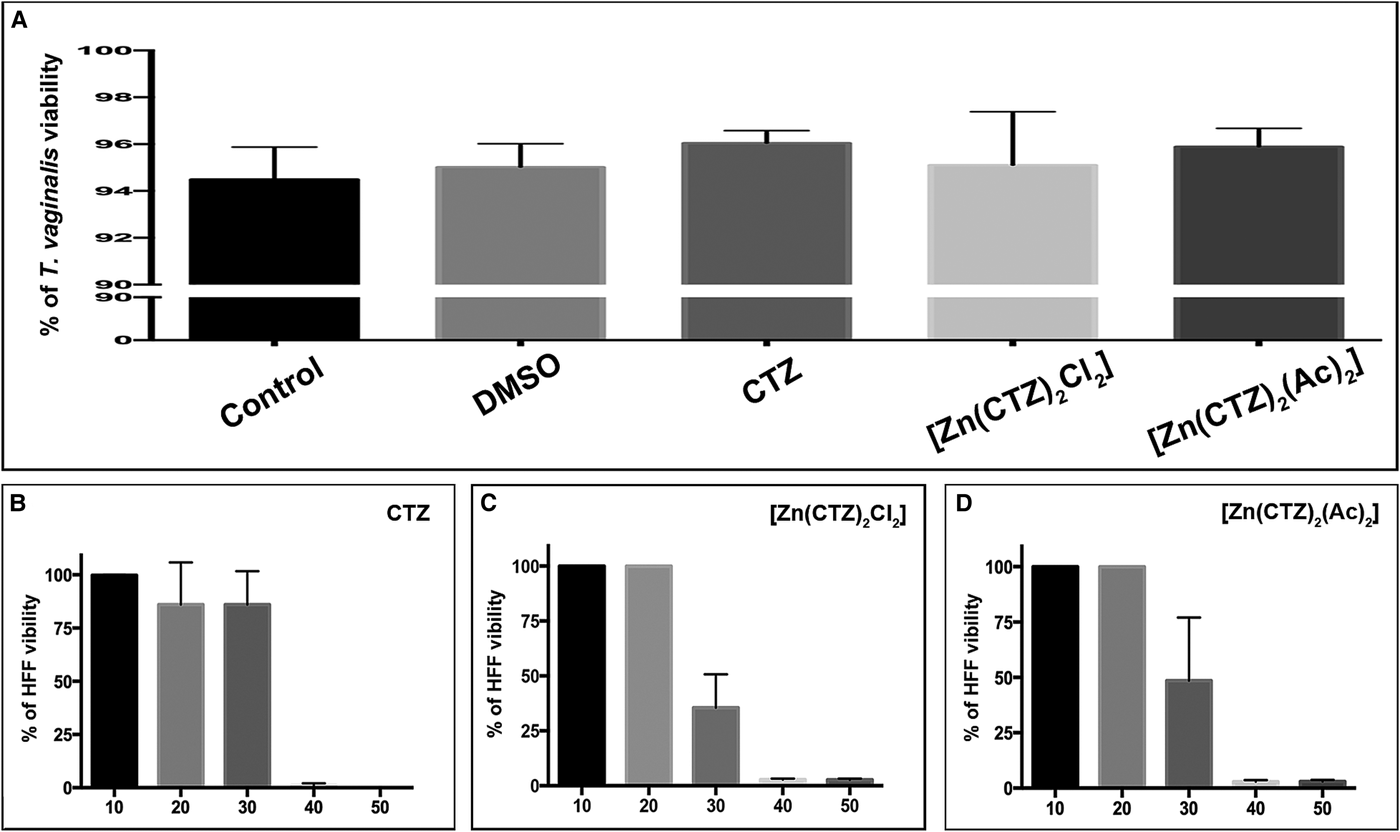
Fig. 9. Cytotoxic effects of clotrimazole and its zinc complexes on cultures in vitro. Quantitative analysis of viability of T. vaginalis using PI (a) and the HFF cell line using MTS (b). (a) Viability analyses of control and treated parasites were performed after 48 h of drug incubation. The following concentrations of the compounds were used: 19 µ m of CTZ, 10.3 µ m of [Zn(CTZ)2Cl2] and 5.1 µ m of [Zn(CTZ)2(Ac)2]. No significant differences in viability are observed. The values are expressed as the mean ± s.d. for each of the three independent experiments performed in triplicate. Fifty-thousand parasites/events per sample were counted by flow cytometry. One-way ANOVA was performed to assess significance (b–d). Treatment of HFF with different concentrations of CTZ (b), [Zn(CTZ)2Cl2] (c) and [Zn(CTZ)2(Ac)2] (d) showed that the compounds were innocuous to human cells at IC50 concentrations, but they become toxic at higher concentrations.
We performed cytotoxicity assays to evaluate the effects of CTZ and its zinc complex compounds against primary cultures of Human Foreskin Fibroblast (HFF) (Fig. 9b–d). Treatment with different concentrations of CTZ and zinc-CTZ complexes (Fig. 9b–d) showed that the compounds did not affect human cells at IC50 concentrations. The Toxic Concentration of 50% (TC50) and the Selective Index (SI) for HFF were 34.7 µ m (SI = 1.8) for CTZ (Fig. 9b), 29.7 µ m (SI = 2.9) for [Zn(CTZ)2Cl2] (Fig. 9c) and 28.4 µ m (SI = 5.6) for [Zn(CTZ)2(Ac)2] (Fig. 9d).
Discussion
For many years, public health policies have neglected trichomoniasis, mainly because of the lack of information regarding the disease and the assumption that is just a nuisance infection. Metronidazole exhibits high efficacy, and at a single 2-g oral dose, the cure rate is 90–95% (Workowski et al., Reference Workowski and Berman2010). Despite the clinical effectiveness of 5-Nitroimidazole compounds, these drugs can lead to resistance against T. vaginalis (Lossick and Kent, Reference Lossick and Kent1991; Schwebke and Barrientes, Reference Schwebke and Barrientes2006). Clotrimazole has been used as an alternative treatment for recalcitrant-Metronidazole-treated infections of T. vaginalis in vitro or in vivo when combined with other compounds (Lossick and Kent, Reference Lossick and Kent1991; Debbia et al., Reference Debbia, Campora, Massaro, Boldrini and Schito1996; du Bouchet et al., Reference du Bouchet, Spence, Rein, Danzig and McCormack1997; Mammen-Tobin and Wilson, Reference Mammen-Tobin and Wilson2005).
Zinc salts have been previously tested against trichomonas in vitro (Krieger and Rein, Reference Krieger and Rein1982; Benchimol et al., Reference Benchimol, Almeida, Lins, Gonçalves and de Souza1993; Figueroa-Angulo et al., Reference Figueroa-Angulo, Rendón-Gandarilla, Puente-Rivera, Calla-Choque, Cárdenas-Guerra, Ortega-López, Quintas-Granados, Alvarez-Sánchez and Arroyo2012; Byun et al., Reference Byun, Jeong, Kim, Lee, Sung and Kim2015). As a result, it has been suggested that the low level of infections detected in males could be related to the urogenital microenvironment, which accumulates a higher concentration of zinc and affects the pathobiology of trichomonads (Byun et al., Reference Byun, Jeong, Kim, Lee, Sung and Kim2015). Indeed, previous work showed that the prostatic glands have a zinc-rich environment that creates adverse conditions for T. vaginalis (Levy and Fair, Reference Levy and Fair1973). Previous studies demonstrated that Zn2+ alters trichomonas virulence, and, as a consequence, affects the morphology and expression of several virulence factors in the prostatic host cells (Vazquez-Carrillo et al., Reference Vazquez-Carrillo, Quintas-Granados, Arroyo, Mendoza-Hernández, González-Robles, Carvajal-Gamez and Alvarez-Sánchez2011; Figueroa-Angulo et al., Reference Figueroa-Angulo, Rendón-Gandarilla, Puente-Rivera, Calla-Choque, Cárdenas-Guerra, Ortega-López, Quintas-Granados, Alvarez-Sánchez and Arroyo2012). In this study, we showed for the first time (1) the ultrastructural alterations caused by clotrimazole in T. vaginalis and (2) the effectiveness of zinc-clotrimazole complexes on the parasites.
We investigated two Zn-clotrimazole complexes and their zinc salts. Our results showed that when the parasites were incubated with IC50 concentrations of clotrimazole and Zn-CTZ compounds, we observed important ultrastructural alterations, but the most severe ultrastructural alterations were seen with the Zn-CTZ complex. We also obtained an IC50 of 4.9 µ m after 48 h of treatment with [Zn(CTZ)2(Ac)2], which shows that the complex with ZnAc2 improved CTZ activity compared to CTZ alone (17.2 µ m). Although [Zn(CTZ)2(Ac)2] is composed by two molecules of CTZ per complex, the IC50 for [Zn(CTZ)2(Ac)2] is 3 times lower than for CTZ alone, and this shows that the activity of CTZ was twice improved in the complex. Metronidazole had IC50 concentrations ranging from 1.5–400 µ m (Upcroft and Upcroft, Reference Upcroft and Upcroft2001; Crowell et al., Reference Crowell, Sanders-Lewis and Secor2003; Goodhew and Secor, Reference Goodhew and Secor2013). In the case of the JT strain used here, it was previously shown that the IC50 concentration of metronidazole was 1.8 µ m (Rocha et al., Reference Rocha, de Andrade Rosa, de Souza and Benchimol2014Reference Rocha, de Andrade Rosa, Urbina, de Souza and Benchimolb).
The combination of metronidazole with other drugs has been extensively tested for the treatment of T. vaginalis (Crowell et al., Reference Crowell, Sanders-Lewis and Secor2003; Goodhew and Secor, Reference Goodhew and Secor2013). The dual administration of zinc sulfate and metronidazole was tested, and the authors observed a decrease in the drug doses needed to treat with success recalcitrant trichomoniasis cases (Houang et al., Reference Houang, Ahmet and Lawrence1997). Here, we synthesized a new compound from two chemicals known to have trichomonicidal effects (clotrimazole and zinc), and both compounds are known to be active against T. vaginalis. We evaluated the effect of a combination of CTZ plus ZnAc2 on parasite growth and compared with the zinc-clotrimazole complex. The IC50 of the combined treatment after 48 h was 15.7 µ m, which very close to the IC50 found for CTZ alone (17.2 µ m). By contrast, the [Zn(CTZ)2(Ac)2] had a better anti-proliferative activity than the combination of CTZ and ZnAc2 and was able to inhibit T. vaginalis with an IC50 of 4.9 µ m. This confirms that the significant improvement in the activity anti-T. vaginalis is exclusively seen when CTZ is coordinated to zinc atom, producing the coordination compound ([Zn(CTZ)2(Ac)2]).
As mentioned above, clotrimazole is reasonably safe without eliciting serious side effects when administrated in humans (Crowley and Gallagher, Reference Crowley and Gallagher2014). We further investigated the effect of CTZ and its zinc derivatives on a human foreskin cell line (HFF) in vitro to assess cell toxicity. We observed that in addition to being more actives, the complexes also had an improvement in selectivity for HFF when compared to CTZ. The Selective Index (SI) values for [Zn(CTZ)2(Ac)2] and [Zn(CTZ)2Cl2] were 2.9 and 5.6, respectively, while SI value for CTZ was 1.8. The SI for [Zn(CTZ)2(Ac)2] was 3.2 times higher than CTZ. Thus both metal complexes are more tolerable for HFF in the effective concentrations than CTZ. This is evident when the IC90 for [Zn(CTZ)2(Ac)2] complex (<20 µ m) is non-toxic for HFF (<30 µ m), while the IC90 for CTZ (>40 µ m) exceeds the toxic concentration for HFF (>30 µ m).
Our results demonstrated that the use of CTZ and its zinc derivatives had no deleterious effects on host cells, suggesting that these compounds may have selective anti-parasitic activity. In the case of metronidazole, there was a reduction in viable cells even at low concentrations (i.e. 1.8 µ m) for HeLa cells (Rocha et al., Reference Rocha, de Andrade Rosa, de Souza and Benchimol2014a; Reference Rocha, de Andrade Rosa, Urbina, de Souza and Benchimolb). Tinidazole is thought to be less toxic in human cells than metronidazole (Crowell et al., Reference Crowell, Sanders-Lewis and Secor2003); however, it was demonstrated that at therapeutic concentrations (0.1, 1, 10 and 50 µg mL−1), tinidazole is genotoxic, cytotoxic and can induce apoptotic cell death in vitro (López Nigro and Carballo, Reference López Nigro and Carballo2008).
Ultrastructural analyses of parasites indicated that the compounds used in the present work induced a number of alterations, such as the appearance of rounded cells, membrane projections and atypical organelles. It is important to highlight that we found two or more of these alterations in the same treated cells. Clotrimazole may disrupt the cell membrane, alters both membrane morphogenesis and the membrane ultrastructure (McGinnis and Rinaldi, Reference McGinnis, Rinaldi and Lorian1991; Debbia et al., Reference Debbia, Campora, Massaro, Boldrini and Schito1996).
Hydrogenosomes are considered the main target organelle of drugs that affect trichomonads (Land et al., Reference Land, Clemens and Johnson2001; Wright et al., Reference Wright, Webb, O'Donoghue, Upcroft and Upcroft2010; Rosa et al., Reference Rosa, Rocha, de Souza, Urbina and Benchimol2011). Sterol biosynthesis inhibitors disrupt hydrogenosomal morphology (Rosa et al., Reference Rosa, Rocha, de Souza, Urbina and Benchimol2011). Parasites treated with metronidazole exhibited alterations in the size of this organelle (Wright et al., Reference Wright, Webb, O'Donoghue, Upcroft and Upcroft2010) and disorganization of hydrogenosomal proteins that act in the organelle's metabolism (Land et al., Reference Land, Clemens and Johnson2001). Zinc treatment targets the hydrogenosomes (Benchimol et al., Reference Benchimol, Almeida, Lins, Gonçalves and de Souza1993) and provokes alterations in hydrogenosomal vesicles, such as changes in electron density and size (Benchimol et al., Reference Benchimol, Almeida, Lins, Gonçalves and de Souza1993). Here, we observed altered hydrogenosomes when zinc-clotrimazole complexes were used, which corroborates previous observations in the literature. These data suggest that [Zn(CTZ)2(Ac)2] might be used to treat trichomonas. This treatment could be performed with intravaginal administration mainly by patients who have some nitroimidazole restrictions, such as nitroimidazole-resistant infections or who are allergic to nitroimidazoles.
In summary, we show that new zinc- clotrimazole complex, [Zn(CTZ)2(Ac)2], is effective and enhanced CTZ activity against T. vaginalis. Although the [Zn(CTZ)2(Ac)2] mechanism of action is still unknown, more experiments are in course to clarify this question. Our results suggest that [Zn(CTZ)2(Ac)2] may constitute a potential and safe compound that could be used as intravaginal treatment for T. vaginalis infections.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118201900043X
Author ORCIDs
Victor Midlej, 0000-0001-9294-6597; Marlene Benchimol, 0000-0002-4957-8538
Acknowledgements
We acknowledge Dr Jan Tachezy from Charles University, Czech Republic for kindly provided the polyclonal antibody anti-pyruvate:ferrodoxin oxidoreductase (PFOR).
Financial support
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Programa de Núcleos de Excelência (PRONEX), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Universidade Federal do Rio de Janeiro (UFRJ) and by Centro Nacional de Bioimagens (CENABIO). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conflicts of interest
There is no conflict of interest.
Ethical standards
Not applicable.