Introduction
Head lice, Pediculus humanus capitis are obligatory bloodsucking ecto-parasites that live on humans’ scalps. Their long-term association with the human host has also become the focus of recent primate and human evolution investigations, because ecto-parasites such as Pediculidae lice closely mirror the evolution of their hosts (Reed et al., Reference Reed, Smith, Hammond, Rogers and Clayton2004; Boutellis et al., Reference Boutellis, Drali, Rivera, Mumcuoglu and Raoult2013). Head lice have accompanied humans since the split of the human lineage from their close cousins, the chimpanzees. Despite jumping from head to head (horizontal transmission) they do not vector human pathogens. According to the circumstances their reproduction can be out of control on some heads, leading to supernumerary infestations (Alexander, Reference Alexander1984). Recent worldwide surveys confirm that both developing and developed countries are equal victims of pediculosis capitis, with the rate of prevalence in school children increasing rapidly and independently of their socioeconomic status (Leung et al., Reference Leung, Fong and Pinto-Rojas2005). Although most statistics and surveys have been conducted on school children (Jahnke et al., Reference Jahnke, Bauer, Hengge and Feldmeier2009; Devore and Schutze, Reference Devore and Schutze2015), older women are also prone to head lice infestations (Maunder, Reference Maunder1983).
Human lice need to complete their whole life cycle on hairy human scalps. The cycle involves different stages, starting with the egg, also known as a nit, followed by the larva or first mobile instar, two nymphal stages and then adults (female and male). The head lice full life cycle takes approximately 24–28 days (Buxton, Reference Buxton1947); very close to a calendar month, giving a total of 12–13 generations a year (on a head with continuous pediculosis). The length of the life cycle is highly stable due to the steady environmental conditions on the human scalp. If the infestation becomes severe there is an overlapping of generations or cycles, which indirectly affects the ability of females to oviposit. Very little is known about the changes in the biology of lice due to overcrowding (Lang, Reference Lang1975). Mobile stages are adapted to hold the hair shaft using the claws on the tarsi of their legs. Body lice (P. h. humanus), a subspecies continuously splitting from head lice populations (Li et al., Reference Li, Ortiz, Fournier, Gimenez, Reed, Pittendrigh and Raoult2010; Veracx and Raoult, Reference Veracx and Raoult2012; Veracx et al., Reference Veracx, Rivet, McCoy, Brouqui and Raoult2012), attach firmly to the fibres of clothes instead of using human hair. Both subspecies can be found on people sustaining severe infestations, with the body lice a direct consequence of a long-term head louse infestation (Alexander, Reference Alexander1984). Head and body lice can be separated by their morphology (Ferris, Reference Ferris1951). The two subspecies also differ in their reproductive behaviour. Female body lice deposit their eggs forming groups or clusters, while head lice do so rarely, and only in severe infestations (Lang, Reference Lang1975; Maunder, Reference Maunder1983). The characteristic pattern of nit distribution in the hair of school children, with regular or occasional infestations, involves a few nits attached to some isolated hairs (Lang, Reference Lang1975; Maunder, Reference Maunder1983; Alexander, Reference Alexander1984). Therefore, formation of nit clusters uniquely happens when female head lice are exposed to crowded conditions. The physical space available to the females for oviposition between nits is compromised in severe infestations, resulting in the clustering of nits and in the monthly overlapping of generations (Alexander, Reference Alexander1984).
Human lice are considered trace evidence in a number of forensic investigations and cases of neglect. Because they are bloodsucking parasites and feed very often [approximately every 2 h (Feldmeier, Reference Feldmeier2017)], their last blood-meal (i.e. the host's blood, rich in mtDNA) becomes reliable evidence in cases of rape or murder. The presence of human lice at a crime scene helps to identify culprits or victims, by matching the human haplotypes in their blood-meal (from inside their gut) with those of the suspects or victims (Lord et al., Reference Lord, DiZinno, Wilson, Budowle, Taplin and Meinking1998; Davey et al., Reference Davey, Casey, Burgess and Cable2007). The association of lice with neglect is long known, although not a topic explored in recent years. Neglect is associated with severe infestations in children, elderly and the homeless (Alexander, Reference Alexander1984; Bennett and Kingston, Reference Bennett and Kingston1993; Beagley and Hann, Reference Beagley, Hann, Hann and Fertleman2016). In most cases, it is a clinician or nurse caring for a patient who discovers and reports the heavy infestation, otherwise it may remain un-reported (Bennett and Kingston, Reference Bennett and Kingston1993; Beagley and Hann, Reference Beagley, Hann, Hann and Fertleman2016; Durand et al., Reference Durand, Andrantsoanirina, Brun, Laroche and Izri2018). The only existing in-use definition and protocol for diagnosing the level of pediculosis dates back to 1977. It was proposed that for a severe pediculosis, a minimum of circa 200 mobile forms is expected, 10% of which have to be females (Maunder, Reference Maunder1977). The latter is not difficult to observe because the sex ratio of head lice is female biased, independently of the level of infestation (Perotti et al., Reference Perotti, Catala, Ormeno, Zelazowska, Bilinski and Braig2004). There have been just a handful of attempts to change the method of assessment of pediculosis. For example, by counting all Pediculus specimens (either nits, adults or nymphs) recorded within a sample area of 2 × 2 cm and assigning a rank to the level of pediculosis, such as low, moderate or high (Gazmuri et al., Reference Gazmuri, Arriaza, Castro, González, Maripan and Saavedra2014). The number of lice will, however, only be informative regarding the level of the infestation and will not provide insights into the time-length of the infestation, either with respect to the history of the pediculosis capitis, or the circumstances surrounding the initial and later stages of infestation. The more data or information collected, the better the chances of detecting and analysing frequent forms of neglect.
The majority of previous studies on neglect did not consider the biology, physiology or behaviour of lice. Infestation rates are generally underestimated (Chosidow, Reference Chosidow2000), mainly due to the lack of a common survey protocol. In this work, a new method for pediculosis capitis diagnosis is proposed to professionals, for consideration of its feasibility and utility in assessment of head lice infestations for medical or forensic analyses. The protocol includes a single page form (Supplementary Materials) to be used when assessing infestations in order to facilitate and speed up diagnosis, to routinely record cases of pediculosis capitis, to build databases and to simultaneously assess the occurrence of neglect. With the aim of defining the most critical parameters to diagnose neglect using head lice infestations, a series of comparative numerical analyses of the two most frequent degrees of pediculosis, regular or moderate (e.g. as typically found in school-children) and severe, were performed. Data analysis especially used nit (egg) numbers, nit-clustering and nit spatial distribution. For the severe infestation, data of P. h. capitis from a case study (Pilli et al., Reference Pilli, Agostino, Vergani, Salata, Ciuna, Berti, Caramelli and Lambiase2016) involving serious neglect followed by death were analysed. Both the results of the numerical analyses and of the cause of death in the case study were interpreted in the light of lice biology, reproduction and oviposition behaviour.
Materials and methods
Summary of the case study, a severe infestation followed by death
The case of a severe infestation which was followed by the death of the victim has been described elsewhere (Pilli et al., Reference Pilli, Agostino, Vergani, Salata, Ciuna, Berti, Caramelli and Lambiase2016). In brief, an elderly woman in a critical condition who was sustaining a massive head louse infestation was received in the emergency ward of a local hospital. The patient died hours after being admitted. She had very long hair, which allowed analyses based on hair growth and nit accumulation over time. An investigation was initiated to establish the level of neglect and the time it had lasted (Pilli et al., Reference Pilli, Agostino, Vergani, Salata, Ciuna, Berti, Caramelli and Lambiase2016). Forensic entomologist SL collected insects and data from hair samples (Pilli et al., Reference Pilli, Agostino, Vergani, Salata, Ciuna, Berti, Caramelli and Lambiase2016), and noted that the medication consumed by the patient was nifedipine, an antihypertensive, which is freely available (not restricted by prescription) in Italy where the victim lived.
Numerical analyses of hairs, nits and clusters from the case study
SL collected lice adults and full-length hairs extracted from all parts of the scalp. The hairs were used for further lice investigation, including estimation of the number of nits as well as clusters of nits, distances between nits and the number of nits/cluster.
Eighty full hairs were collected. To determine total nits/hair, 41 hairs were analysed. Nit-cluster analyses were undertaken on 10 of these 41 hairs, which presented up to 20 continuous and crowded nit clusters. A total of four hairs (of the 41) were also used to count the total number of nits and clusters for an estimated period of 12 months, with a month considered equal to 1 cm of hair length (Lapeere et al., Reference Lapeere, Brochez, Vander Haeghen, Mabilde, Vander Stichele, Leybaert and Naeyaert2005).
Numerical analyses of regular infestations and comparison of infestations
Nits counted on 20 consecutive attachment sites in the severe infestation and whole hairs in regular infestations, were used to compare attachment distances and formation of clusters. Data of regular infestations (occasional, typical of school children) were provided from previous projects (Perotti et al., Reference Perotti, Catala, Ormeno, Zelazowska, Bilinski and Braig2004). For comparison purposes, four hairs of regular infestations were included (Perotti et al., Reference Perotti, Catala, Ormeno, Zelazowska, Bilinski and Braig2004). Growth curves of monthly accumulated values were built with the log10 of nit or cluster numbers. The use of log10 allowed better visualization of extreme values, such as occurred in severe infestations.
Nits within a cluster all belong to one generation, they are oviposited by a close cohort of females (Lang, Reference Lang1975). There is a minimum physical space needed by females to manoeuvre to properly deliver and attach eggs to the hair shaft. In this study, this biological space is defined as the spatial distance required by a gravid female to hold the hair during oviposition using both the tarsal claws and gonopods for attachment. If a female louse is not provided with this minimum ‘biological space’ for oviposition, it would likely glue itself to the hair shaft, together with the egg and die in situ. Both parts of the female body, tarsi and gonopods, therefore, need access to an unoccupied, specific length of hair-shaft.
Statistical analyses
Statistical analyses used W (Shapiro-W) and Wilcoxon for normality tests in PAST3 (Hammer et al., Reference Hammer, Harper and Ryan2001) and Microsoft Excel 2013 for descriptive parameters and correlations.
Microscopic analysis of female lice reproductive organs
Oviducts and ovaries of 10 gravid females were dissected using a stereo microscope (Leica M125) and inspected for their quality and state of development using a phase contrast microscope (Leica DMLB). The females, as well as a few removed developing nits, were kept in 75% (v/v) ethanol. Before inspection, they were rehydrated in PBS [phosphate basic (Na) solution] (Perotti et al., Reference Perotti, Allen, Reed and Braig2007), as rehydration smooths the tissues and restores a ‘living’ appearance. Despite the hydration treatment, ovaries and nits were very damaged and fragile.
Development of a new protocol for assessing pediculosis capitis
A comprehensive literature review, addressing early methods of evaluation of head lice infestations, such as counts of eggs or mobile stages guided the layout of the new method of assessment presented here (Roy and Ghosh, Reference Roy and Ghosh1944; Buxton, Reference Buxton1947; Lang, Reference Lang1975; Maunder, Reference Maunder1977, Reference Maunder1983; Alexander, Reference Alexander1984; Perotti et al., Reference Perotti, Catala, Ormeno, Zelazowska, Bilinski and Braig2004; Leung et al., Reference Leung, Fong and Pinto-Rojas2005; Devore and Schutze, Reference Devore and Schutze2015; Beagley and Hann, Reference Beagley, Hann, Hann and Fertleman2016). The new method incorporates information generated from lice biology and from the analysis of the case study described above, plus the comparison of the two most common levels of infestation. It includes a new survey form, supplied in Supplementary Materials.
Availability of data and material
All data used in the numerical analysis of lice and nits is provided in this manuscript (main text and Supplementary Materials); a few lice specimens of the case studied are deposited at the collection of one of the authors, Dr Perotti's Laboratory, University of Reading. No human hair was saved (Pilli et al., Reference Pilli, Agostino, Vergani, Salata, Ciuna, Berti, Caramelli and Lambiase2016).
Results
Case study's louse population
All the hairs of the victim of neglect followed by death were entangled and the majority glued to each other, particularly at the occipital area of the scalp, showing a plica polonica formation.
Lice collected during the autopsy were all dead at the time of sampling. From a collection of 200 mobile specimens, over 40% were adults (N Adults = 79) and of this, >50% females (N Females = 41), indicating a high level of infestation and confirming the expected female bias. Skin bite-marks as well as dead specimens were numerous on the upper parts of the thorax of the victim presenting a gradient of infestation (bite marks) decreasing downwards to the waist. There was no possibility of examination or search for nits laid or attached inside the garments the victim was wearing, therefore, it is not possible to rule out an ongoing transition towards body lice development.
There was not a single hair without attached nits, resulting in a 100% prevalence of infestation from hairs extracted from different parts of the scalp (N Hairs = 80). All observed hairs showed a pattern of chained nit attachment arranged in clusters formed of a varied number of nits.
The average length of hair that was totally covered with nits (or clusters) was 69 mm (±19.5) and the intensity of attached nits resulted in a median of 65, varying between a minimum of 33 to a maximum of 104 nits/hair (Table S1). This approximates to one nit for every millimetre of hair, although the main observed pattern was that of clustering of the nits.
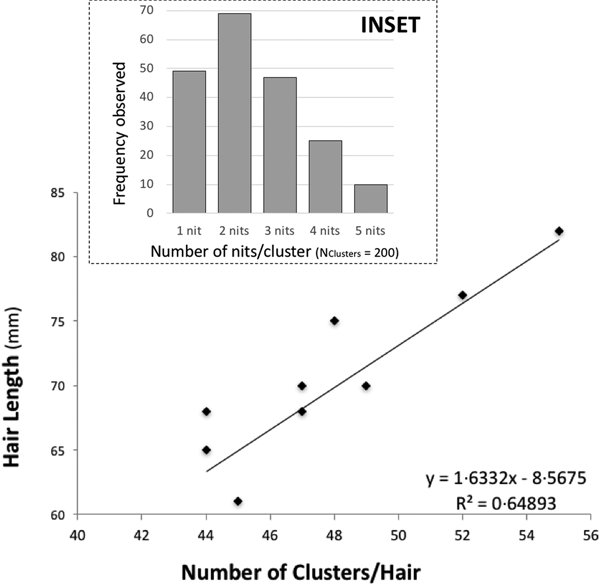
There is some evidence of correlation between the (total) length of the hair and the number of clusters (N Hairs = 10) (Fig. 1). Nit-clusters were formed by two to five nits that were overlapping on their cemented-attachment site over the length of the hair (Fig. 1, inset).
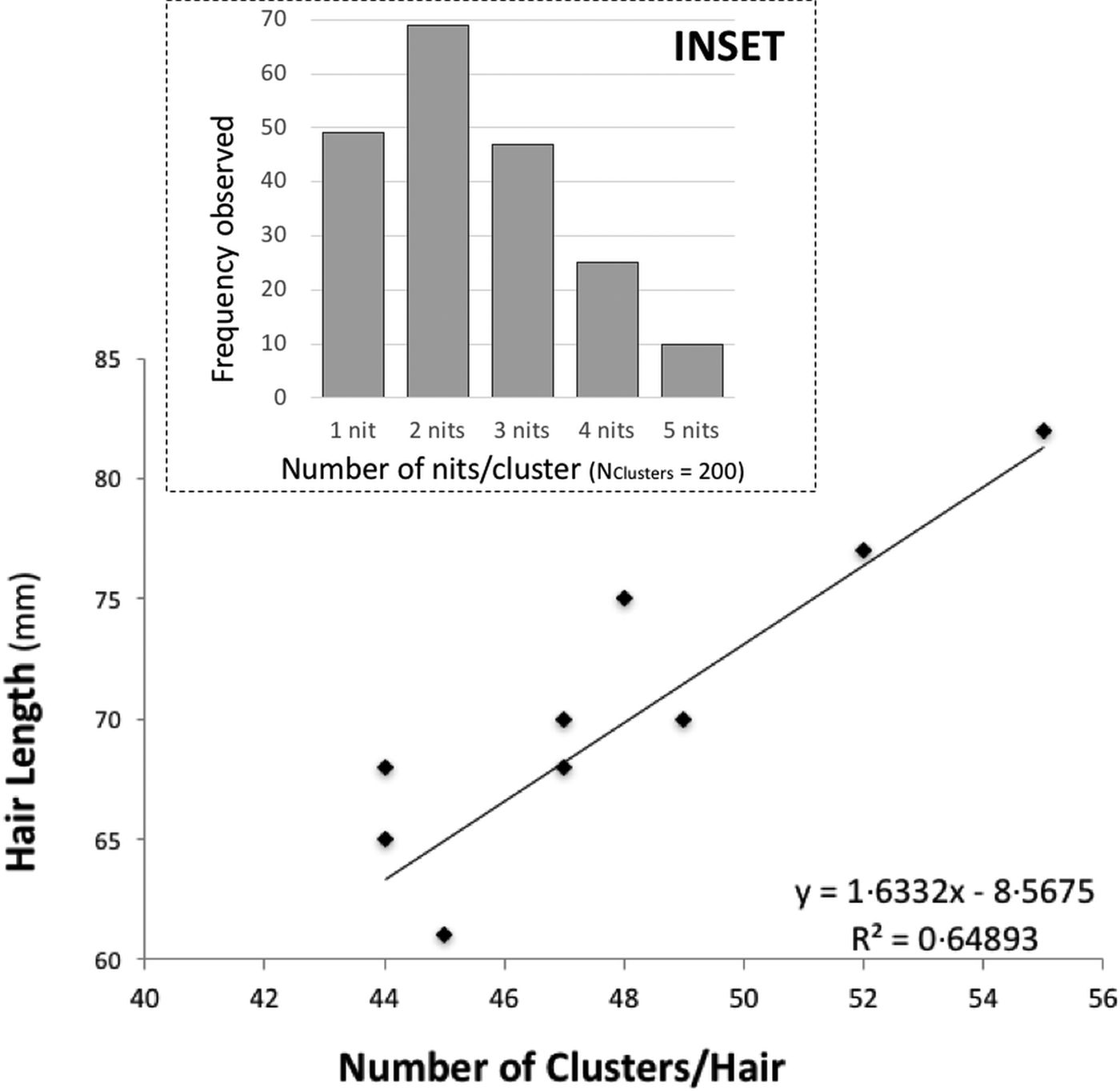
Fig. 1. Main graph: distance between nit-clusters over a length of hair covered with nits. Nit-cluster coverage with a maximum length between 60 and 85 is shown, the longer the hair shaft, the more clusters there are. A positive correlation is shown, confirming the maintenance of a minimum distance between nits, needed by females to manoeuvre for oviposition. Inset: frequency of nits in the clusters, the largest clusters containing five nits are the least frequent.
Cluster intensity followed a normal distribution over the hairs (W = 0.963, P = 0.199), averaging 47 clusters/hair (±19.5) (with the same median value, of 47 clusters/hair). For the nit content of the clusters, the minimum median value was two and the maximum three nits/cluster (N Clusters = 200). However, 10 clusters contained five, 49 contained only one, while the vast majority contained two, followed in abundance by three and four nits (Fig. 1, inset).
Despite their homogeneous appearance on the hairs, the distance between nit-clusters did not follow a normal distribution (W = 0.908, P < 0.003), with an average separation of 1.66 mm (±0.55) between attachment sites.
Female lice averaged 2.23 mm in length (N Females = 18), and the ‘biological distance’ between tarsi and gonopods averaged approximately half of the total body length, 1.27 mm (Fig. 2). Each cluster covered a linear distance of 2–4 mm, depending on the extension of the cement/cluster and on the number of nits clustered (Fig. 2, inset).

Fig. 2. Position of the female louse over the hair shaft at oviposition. A clear shaft length of 2.25–2.5 mm allows the female to position its body for the correct attachment of a nit. In crowded conditions this space reaches a minimum ‘biological space’. Inset (top right): close up of nit-clusters in the crowded habitat of the severe infestation, clusters of two and three nits are shown.
Comparisons between severe (case) and regular (school children) infestations
Analysis of population growth of the nits allowed a more comprehensive characterization and assessment of severe vs occasional infestations.
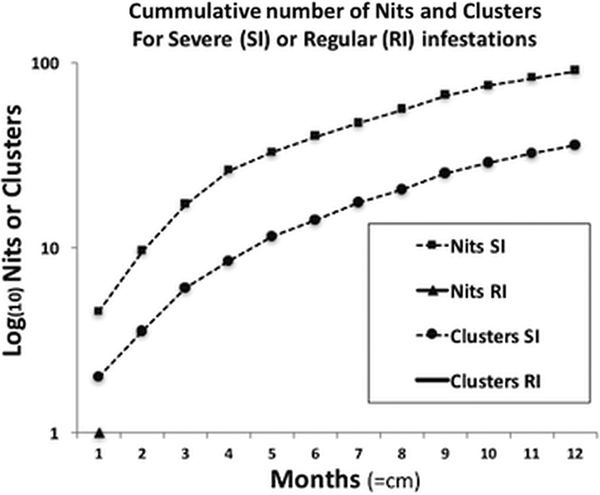
For the severe infestation, the monthly accumulated number of nits and clusters showed a uniform growth pattern (normally distributed), reaching a plateau of intensity. This pattern significantly contrasted with the population growth from the occasional infestations, where there was no population or only small deme formation [medianSevere = 163 and medianRegular = 1 (W = 78, P < 0.01)] (Fig. 3).
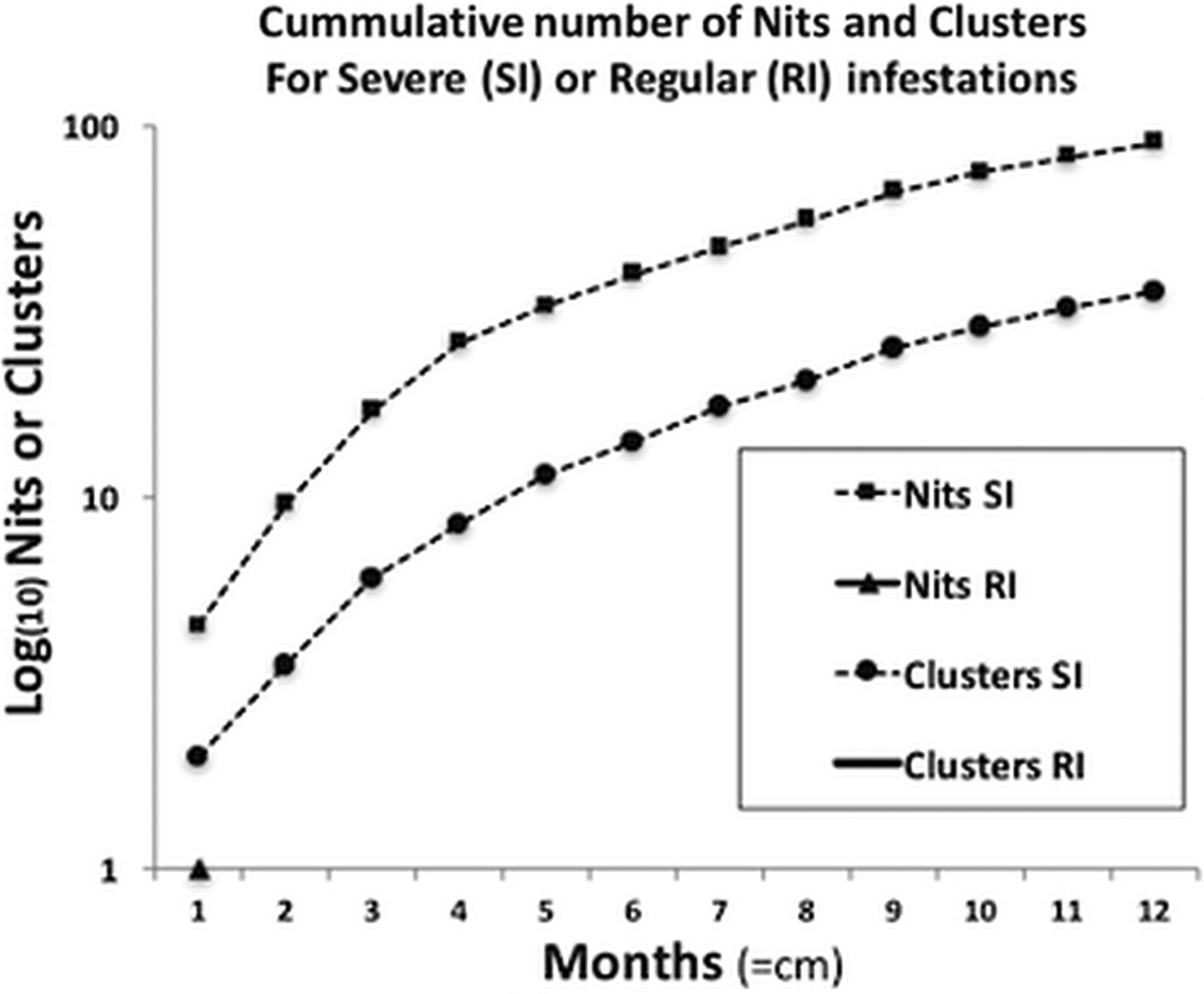
Fig. 3. Comparison of the progressive accumulation of nits and clusters between severe and regular infestations. The two curves shown belong to the severe infestation, presenting a sustained growth reaching almost a plateau of saturation, or maximum growth, due to limited space. In terms of clusters, in regular infestations neither persist, reproduce nor grow, hence no data is shown (no curves, full lines or triangles).
Case study: hair growth, nits and consumption of medicine
It was not possible to know the exact hair growth rate of the host, therefore, it is not advisable to estimate duration of infestation solely by measuring the length of hair carrying nit-clusters. However, it was possible to estimate time of continuous infestation using nit-cluster coverage and number of generations. The maximum nit-cluster linear distance recorded on a hair shaft reached 113 mm (N Hairs = 41). Based on an analysis of nit-cluster distribution on hairs using the minimum ‘biological space’ and including a minimum monthly overlap of two generations (two clusters = two generations/month), a suggested time of continuous infestation of 24 (±4) months was estimated. Taking into account rather that one generation involves approximately 24–28 days, a continuous severe infestation dating back between 20 and 28 months, approximately 2 years from the time of death, is suggested. In addition to the reduced biological distance found between clusters, a few adult females or their reproductive organs were found glued to the hair shafts. The presence of sporadic or repeated infestations (of a few isolated clusters) in the oldest (distal) part of the hair shafts was also noted.
A highlight of the analysis of full-length hairs was the ‘unexpected’ gap of clear shaft, carrying no nits, for a short distance at the shafts’ root. The clear length of shaft between the scalp and the first nit, starting from the hair root, showed an average length of 1.45 cm (±0.47) (Fig. 4 and Table S2) with a borderline normal distribution (W = 0.969, P = 0.0503). This could be the result of a sampling artefact, due to the number of hairs used (either too many or too few) but may also suggest that, despite a massive record of living adults on the hairs, there was a total lack of oviposition for a period of up to 2 months.
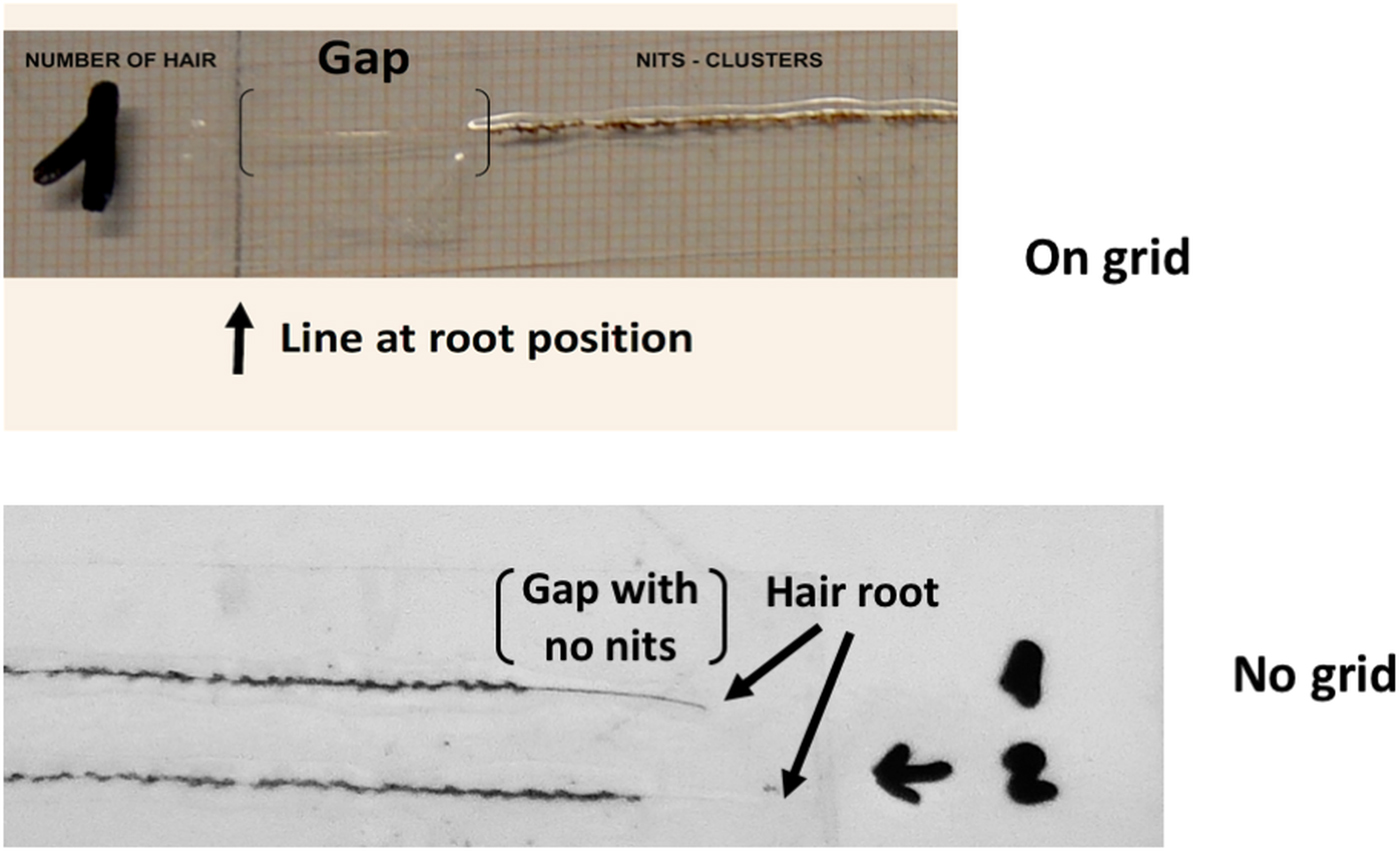
Fig. 4. Hair shaft gap lacking nits, above the root of the hair samples. The absence of nits is a consequence of the interruption in lice reproduction due to the highly concentrated CCB in the blood of the host, which arose from the victim's over consumption or overdosing. On grid (top photo). One hair, the root is indicated as ‘Line at root position’ and the nit-free ‘Gap’ is shown between the brackets. The shaft at the root is not visible due to its transparency. No grid (below). Two hairs, numbered 1 and 2. The roots are indicated by arrows and the nit-free ‘Gap’ corresponds to the position of the brackets.
Observations of the reproductive system of gravid females indicated the presence of interrupted, degenerated or terminated oogenesis. In general, either there were big but deteriorated eggs already shrinking inside their mothers, or there was an absence of mature oocytes (=ready formed nits). In addition to the inspected adults, there were a small number of larvae carcasses disintegrating in the samples examined and a similar number of first nymphal stages. This is further evidence of reproductive failure, where females were not able to lay their eggs and the eggs died inside the mothers; this seems to have occurred for a period of up to 2 months, right before the host's death.
New assessment method of pediculosis capitis
The ‘Pediculosis-capitis Survey Form’ (Supplementary Materials) incorporates a few, new, but rigorous criteria for facilitating decision making. The criteria are described in Supplementary Materials and are summarized in the assessment form.
The criteria for assessment include: estimated number of infested hairs; location or position of nits/clusters on the head; number of nit attachment sites; nit-cluster formation and distance between clusters. Other features to consider include, for example, the origin of the louse infestations. This criterion includes a literature review of body lice and their vector capacity, highlighting cases of re-emerging infectious diseases, especially from the recent arrival of immigrants in Europe.
Discussion
The use of just the number of mobile lice at a particular stage, e.g. adults, as a sole element to analyse pediculosis, does not provide detailed information on its history, on neglect, on medical condition or on the cause of death. This work emphasizes the importance of nit-clustering for unravelling details about the circumstances of neglect that otherwise may be overlooked. Therefore, to characterize the level of a head louse infestation, the distribution pattern of nit clusters on a few sampled hairs must be considered.
From the interpretation of the results for nit-clustering and their arrangement on the hair-shaft it is clear that for a certain period there was a lack of space for female oviposition manoeuvres. This implies highly crowded conditions typical of a severe infestation. In a severe infestation, females struggle to find the space required to manoeuvre oviposition as they can only grab and hold empty hair shafts and not nits (there is no morphology/adaptation for this). Even if the intense infestation only covers 2 cm of the hair shaft, and there is ~1 mm of space between small nit clusters covering these 2 cm, this confirms that the patient was neglected for a period of time (2 cm approximates to 2 months). Using hair growth (when possible) to estimate the time when the patient was exposed to neglect can help to interpret the circumstances and time of neglect, e.g. indicating when it happened, or when it started. For example, a patient might be a victim of neglect for only 2–3 months, and this might have started 6 months before its discovery. If his/her hair grows at a rate of 1 cm month−1, the cluster formation should be found ~3.5 cm from the scalp and should cover at least 2 cm of hair-shaft. Over these 2–3 cm of nit-attachments, the clusters should be separated by at least 1 mm, as that is the female's minimum required space for grabbing the hair to oviposit. The use of hair growth has to consider the age of the patient/victim, the older the person the slower the growth.
Nit-clusters were slightly and unevenly distributed on the shafts (non-normally distributed), which could be due to the expected intra-population body size variation of gravid females. The average distance of 1.66 mm (±0.55) between attachment sites clearly suggests, however, that there was no room for extra manoeuvres. The immediate consequence of crowdedness is the overlapping of generations in ovipositing sites (here called clusters), a feature already observed by early researchers (Buxton, Reference Buxton1947), but not considered important until now. The predominance of two-nit clusters followed by three-nit clusters (showing a Poisson distribution, Fig. 1, inset) might also indicate overlapping of a number of generations or group oviposition or even a few demes becoming body lice. Females of body lice are gregarious and nits are deposited in groups in the same area of cloth shared by several females (Veracx et al., Reference Veracx, Rivet, McCoy, Brouqui and Raoult2012).
Based solely on the oviposition behaviour of lice, the estimated length of time of the continuous severe infestation suggests a sustained neglect of approximately 2 years in duration. This represents a clear case of neglect of an elder (Bennett and Kingston, Reference Bennett and Kingston1993). Furthermore, the presence of intermittent infestations, of a few consecutive clusters in the distal or older parts of the hair shafts, suggests that neglect was likely experienced repeatedly, even before the 2 year period of intense and out of control infestation built-up.
The victim of neglect presented a plica polonica, a characteristic feature of gross head lice infestations, confirming neglect (Alexander, Reference Alexander1984). In this condition, the hairs are glued into a sticky secretion and emanate a particular spoiled vinegar smell. Plica polonica was common in Poland, first appearing in the 1200s, when it was described: then it was already a sign of poor hygiene (Brzezinski et al., Reference Brzezinski, Chang, Fan, Krishnan, Peh, Francès, Davatchi and Chiriac2016). In this case study, the doctors and nurses treating the patient noticed the plica formation, as well as the huge louse population extending to the torso (Connor et al., Reference Connor, Selby and Wanat2016).
Unfortunately, it was not possible to sample lice from the torso area or its clothes to confirm the presence of the two P. humanus subspecies. Finding body lice together with a gross head lice infestation on one human host is not novel. Head lice evolving into body lice have been documented on the same individual, particularly homeless people (Veracx et al., Reference Veracx, Rivet, McCoy, Brouqui and Raoult2012). The adaptation to nest in clothing can only occur in head lice of mtDNA Clade A (Li et al., Reference Li, Ortiz, Fournier, Gimenez, Reed, Pittendrigh and Raoult2010). At present, however, there is no phenotype or morphotype available to identify mitochondrial clades. Clade A has a worldwide distribution and is predominant in European countries and it is likely that it was carried by the patient. The transition from head to body lice on a person can only be achieved following a very long-term exposure and continuous infestation (Veracx et al., Reference Veracx, Rivet, McCoy, Brouqui and Raoult2012) and this is another clear sign of a patient being a victim of neglect and of having suffered a long-term, severe pediculosis capitis.
Of particular interest was the finding of a gap at the root of all the hair shafts examined, characterized by the absence of nits from the root-end of the shaft to the last nit oviposited onto it. With an average length of 1.45 cm (±0.47) of nit absence, this represents an abnormal situation for lice biology. Head lice gravid females oviposit circa 5 mm from the host scalp (Roy and Ghosh, Reference Roy and Ghosh1944; Buxton, Reference Buxton1947; Lang, Reference Lang1975; Lapeere et al., Reference Lapeere, Brochez, Vander Haeghen, Mabilde, Vander Stichele, Leybaert and Naeyaert2005). This distance cannot be modified, it is a physiological requirement for the proper development of the embryo. Nits depend on a temperature gradient determined by the distance, also measured in time (as the hair grows), from the scalp producing heat (Buxton, Reference Buxton1940, Reference Buxton1947; Alexander, Reference Alexander1984). The estimated length of the gap with no nits suggests a time lapse of ~2 months and it was found despite many thousands of mobiles, particularly females, crawling on the head. Lice larvae and first instar nymphs (the youngest stages) were very rare and gravid females’ internal or not yet laid oocytes were found to be degenerating or terminated (shrunk) (numbers not collected). This was a clear indication that extrinsic factors were affecting lice reproduction.
The medication consumed by the patient, nifedipine, is used for the treatment of high-blood pressure. This substance actively blocks the movement of calcium through calcium channels, being a well-known calcium channel blocker (CCB). The active ingredient is almost fully attached to proteins in plasma, and metabolized to inactive compounds with a half-life of up 4 h (Hilal-Dantan and Brunton, Reference Hilal-Dantan and Brunton2014). This makes the drug available in blood for several hours, providing a plentiful supply at variable doses in lice blood meals. This drug can be purchased without prescription in Italy, and the patient was able to buy and consume it as desired.
In most sexually reproducing animals, egg activation is induced by the process of fertilization. The sperm mediates a continuous release of intra-oocyte calcium which allows the completion of meiosis and the development of the vitelline membrane to prevent access by more sperm. In this context, arthropods are more flexible than other animals, with sperm being no longer the exclusive trigger of egg activation. Insects, such as lice, use intra-oocyte calcium waves occurring while the eggs or oocytes move through the female's oviducts (Perotti et al., Reference Perotti, Allen, Reed and Braig2007; Horner and Wolfner, Reference Horner and Wolfner2008; Kaneuchi et al., Reference Kaneuchi, Sartain, Takeo, Horner, Buehner, Aigaki and Wolfner2015).
In the discussed case of severe neglect, lice were feeding for years off a host who was on a regular daily consumption of nifedipine, but blood with these moderate daily doses of nifedipine seems not to have affected lice reproduction for about 2 years. However, approximately 2 months before the patient was admitted to hospital and died, lice reproduction had become seriously compromised and stopped. A massive population of lice unable to reproduce suggests an overdose of nifedipine by the victim, powerful enough to stop egg activation and lice development. Lice reproductive behaviour enables us to estimate about 45–60 days prior to death as the time when their neglected host started to exceed safe or recommended doses of nifedipine.
Lice biology indicates a complex case of neglect, initially involving family abandonment (Pilli et al., Reference Pilli, Agostino, Vergani, Salata, Ciuna, Berti, Caramelli and Lambiase2016). The sustained parasitosis lasted for about 2 years and was followed by self-inflicted neglect in the form of self-overdosing of medication, a well-known reaction in abandoned elders (Bennett and Kingston, Reference Bennett and Kingston1993; Burnett et al., Reference Burnett, Clark, Abada, Parker, Flores, Pace-Murphy, Day, McCarthy and Fitzpatrick2018; O'Connor, Reference O'Connor, Day, McCarthy and Fitzpatrick2018).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018002007.
Author ORCIDs
M. Alejandra Perotti 0000-0002-3769-7126.
Acknowledgements
We thank PhD student Jasdeep Rai for the sketches of a louse and nits (from a photograph provided by MAP) in Fig. 2; and Dr Henk Braig for help translating German literature. We would like to thank the Erasmus Staff Mobility scheme between the Universities of Reading (UK) and Pavia (Italy) that allowed MAP and SL to meet and discuss this work.
Author contribution
SL collected the data, including photographs, contributed to the design of the study and revised a final version of the manuscript. MAP designed the study, performed the data analysis, graphs, interpretation of the results and has written the manuscript.
Financial support
MAP research is supported by the BBSRC, Project Reference BB/N001443/1.
Conflict of interest
None.
Ethical standards
Not applicable.