Article contents
Unique genetic structure of the human tapeworm Dibothriocephalus latus from the Alpine lakes region – a successful adaptation?
Published online by Cambridge University Press: 16 May 2022
Abstract
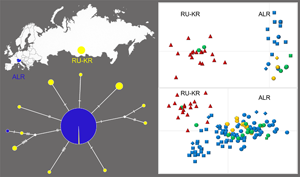
Dibothriocephalus latus is the most frequent causative agent of fish-borne zoonosis (diphyllobothriosis) in Europe, where it is currently circulating mainly in the Alpine lakes region (ALR) and Russia. Three mitochondrial genes (cox1, cob and nad3) and 6 microsatellite loci were analysed to determine how is the recently detected triploidy/parthenogenesis in tapeworms from ALR displayed at the DNA level. A geographically distant population from the Krasnoyarsk Reservoir in Russia (RU-KR) was analysed as a comparative population. One or 2 alleles of each microsatellite locus was detected in plerocercoids from RU-KR, corresponding to the microsatellite pattern of a diploid organism. In contrast, 1–3 alleles were observed in tapeworms from ALR, in accordance with their triploidy. The high diversity of mitochondrial haplotypes in D. latus from RU-KR implied an original and relatively stable population, but the identical structure of mitochondrial genes of tapeworms from ALR was probably a consequence of a bottleneck typical of introduced populations. These results indicated that the diploid/sexually reproducing population from RU-KR was ancestral, located within the centre of the distribution of the species, and the triploid/parthenogenetically reproducing subalpine population was at the margin of the distribution. The current study revealed the allelic structure of the microsatellite loci in the triploid tapeworm for the first time.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press
Footnotes
Equal contribution of both authors.
References
- 7
- Cited by



