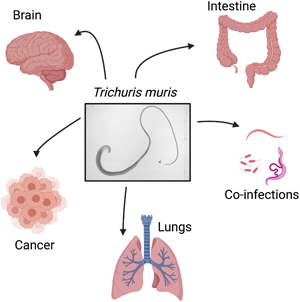
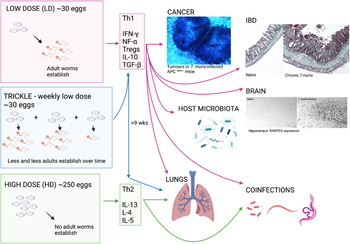
Introduction
Trichuris muris is a mouse intestinal parasitic nematode used as an experimental model for the human counterpart, T. trichiura. This nematode is one of the four major soil-transmitted helminths that infect 1.5 billion people worldwide causing significant morbidity (WHO, 2020). These diseases bear a huge impact on the quality of life of infected people and on the economic growth of infected communities (Hotez et al., Reference Hotez, Alvarado, Basáñez, Bolliger, Bourne, Boussinesq, Brooker, Brown, Buckle, Budke, Carabin, Coffeng, Fèvre, Fürst, Halasa, Jasrasaria, Johns, Keiser, King, Lozano, Murdoch, O'Hanlon, Pion, Pullan, Ramaiah, Roberts, Shepard, Smith, Stolk, Undurraga, Utzinger, Wang, Murray and Naghavi2014).
T. muris inhabits the large intestine and caecum of the host, with adult parasites living with their anterior half tunnelled into the host epithelium and their posterior free in the lumen to facilitate egg deposition (Cliffe and Grencis, Reference Cliffe and Grencis2004). The immune response to T. muris in mice is very well characterized and there is a distinct polarization of immune response in resistant and susceptible strains of mouse (Else and Grencis, Reference Else and Grencis1991; Else et al., Reference Else, Hültner and Grencis1992). Resistant animals produce high levels of interleukin 13 (IL-13) and associated T helper type 2 (Th2) cytokines in response to infection (Fig. 1), which are essential for parasite expulsion via mechanisms such as epithelial cell turnover and mucin production and muscle contraction (Khan et al., Reference Khan, Richard, Akiho, Blennerhasset, Humphreys, Grencis, Van Snick and Collins2003; Cliffe et al., Reference Cliffe, Humphreys, Lane, Potten, Booth and Grencis2005; Hasnain et al., Reference Hasnain, Wang, Ghia, Haq, Deng, Velcich, Grencis, Thornton and Khan2010; Chen et al., Reference Chen, Luo, Li, Kim, Stewart, Urban, Huang, Chen, Wu, Chesler, Trinchieri, Li and Wu2021). In contrast, a susceptible animal produces high amounts of interferon-γ (IFN-γ) and Th1 associated cytokines (Fig. 1) that leads to chronic infection, enabling the parasite to establish to maturity within the large intestine and release eggs into the environment, thereby perpetuating infection. Trickle infections can also be used to more closely mimic a natural infection of repeated low-dose exposures. Weekly trickle infections promote an initial Th1 response but this changes to a dominant Th2 response after 9 weeks (Fig. 1), which prevents any further establishment of worms (Glover et al., Reference Glover, Colombo, Thornton and Grencis2019). Chronic infection, either in genetically susceptible mice or due to a low-dose infection and its associated Th1 response, are associated with dysregulation within the gut, such as crypt hyperplasia and apoptosis (Cliffe et al., Reference Cliffe, Potten, Booth and Grencis2007) together with a regulatory response that is required to limit worm-driven pathology (D'Elia et al., Reference D'Elia, Behnke, Bradley and Else2009; Grencis et al., Reference Grencis, Humphreys and Bancroft2014; Duque-Correa et al., Reference Duque-Correa, Karp, McCarthy, Forman, Goulding, Sankaranarayanan, Jenkins, Reid, Cambridge, Ballesteros Reviriego, Müller, Cantacessi, Dougan, Grencis and Berriman2019). Interestingly, reducing T regulatory (Treg) cells early on during a low-dose infection does have a small but significant effect on the capacity to expel parasites and subsequently intestinal pathology is reduced, suggesting that this induced Treg response is of benefit to both the host and to the parasite (Sawant et al., Reference Sawant, Gravano, Vogel, Giacomin, Artis and Vignali2014). However, this effect on parasite expulsion was lost if Tregs were depleted once infection had become established (Sawant et al., Reference Sawant, Gravano, Vogel, Giacomin, Artis and Vignali2014). A key cytokine produced by CD4+ T cells IL-10, is critical in host survival during T. muris infection (Schopf et al., Reference Schopf, Hoffmann, Cheever, Urban and Wynn2002) although whether Tregs are the major source of IL-10 during T. muris infection is unclear. TGF-β is another regulatory cytokine that is produced during T. muris infection that can dampen CD4+ T cell responses (Li and Flavell, Reference Li and Flavell2008). As with the effects of an early reduction in Tregs, early ablation of TGF-β during a low-dose infection again caused a significant, although partial, reduction in worm numbers (Worthington et al., Reference Worthington, Klementowicz, Rahman, Czajkowska, Smedley, Waldmann, Sparwasser, Grencis and Travis2013). When the ability of dendritic cells to induce TGF-β was prevented, mice were able to clear a low-dose infection efficiently although this did not seem to be dependent upon the generation of Tregs (Worthington et al., Reference Worthington, Klementowicz, Rahman, Czajkowska, Smedley, Waldmann, Sparwasser, Grencis and Travis2013). Thus, it appears that the regulatory response generated by T. muris is complex and involves CD4+ T cells, Tregs, IL-10 and TGF-β contributing to the net result of a chronic infection with controlled intestinal inflammation. This review will discuss the differing effects that either low-dose or high-dose intestinal T. muris infection can have on both enteral and systemic responses in the host (Fig. 1).
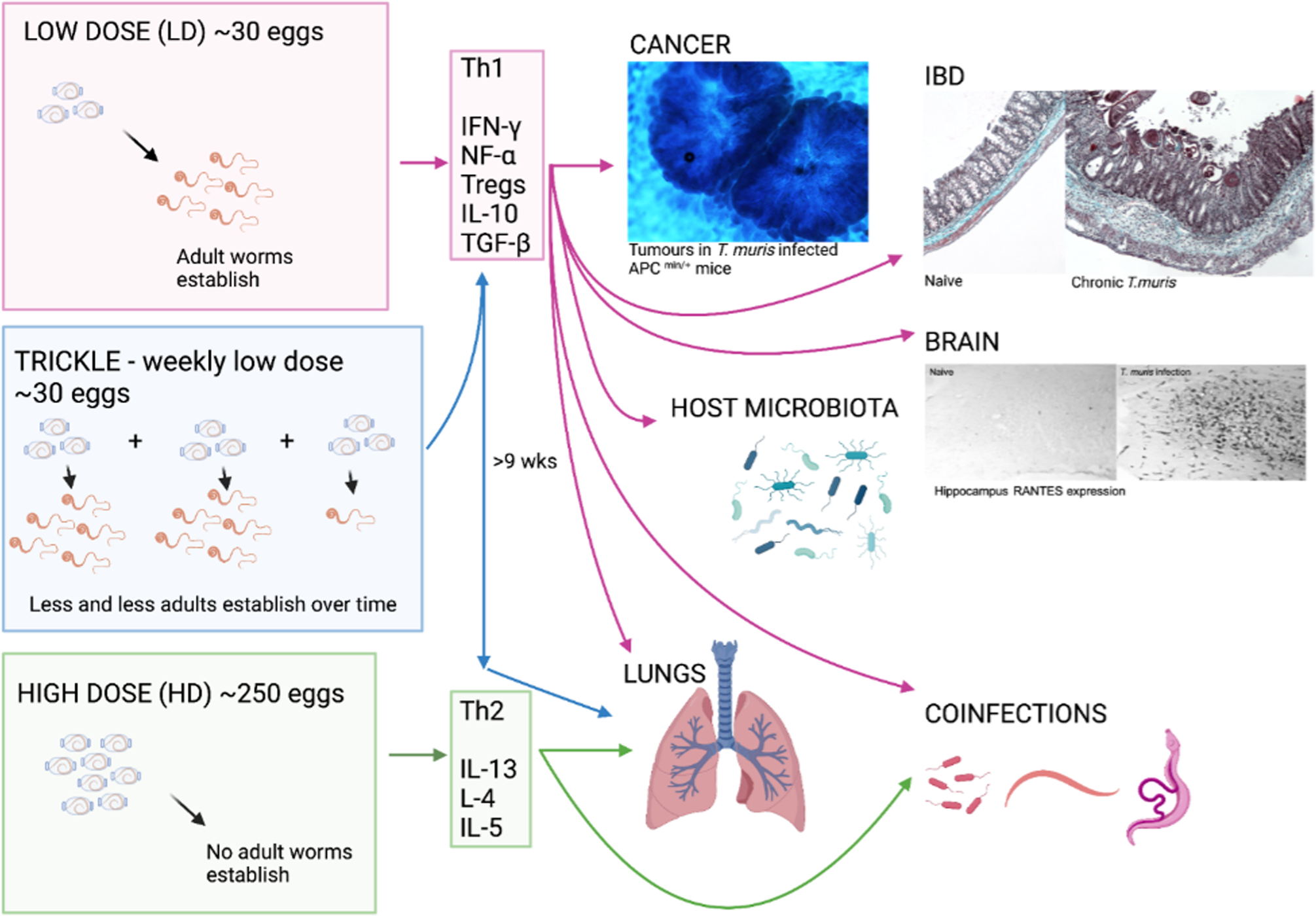
Fig. 1. The whipworm T. muris, though caecal dwelling, can affect many other systems in the body. The immune response to T. muris is dose-dependent with different cytokines being produced in response to the different doses of eggs given which can lead to chronic infection (Th1) or expulsion (Th2). Each of the immune responses to the differing doses of eggs can impact different systems in the body as depicted by the arrows. As pictured, tumours are increased in size and number in a cancer model with chronic T. muris, pathology is increased in chronic infection and shows similarity to IBD, and hippocampus RANTES expression is increased with chronic T. muris infection. Changes in microbiota, lung effects and effects on other infections are also apparent with T. muris infection. (Created with BioRender.com)
Intestinal inflammation
Inflammatory bowel disease (IBD) in humans represents broadly two distinct immunological conditions; Crohn's disease and ulcerative colitis. Disease onset is prompted in genetically susceptible individuals by atypical responses to microbiota or environmental cues such as diet and stress (Guan, Reference Guan2019). The influence of human trichuriasis upon IBD has received little attention, with notable exceptions (Broadhurst et al., Reference Broadhurst, Leung, Kashyap, McCune, Mahadevan, McKerrow and Loke2010). This study followed pathological and immunological changes in an individual with ulcerative colitis prior to and following self-treatment with T. trichiura. The data supported a modulatory role for whipworm infection upon disease severity with infection associated with disease remission. Due to the intestinal niche that Trichuris species inhabit, an effect upon inflammatory disease of the large intestine in the host might be expected. Mechanistically this can be explored in the mouse using T. muris together with murine models of IBD. It is plausible that T. muris infection may cause IBD symptoms while the host immune response to the parasitic infection could have implications on progression of intestinal inflammation. Specifically, it is known that a low-dose infection of ~20 T. muris eggs will proceed to chronicity (Fig. 1), even in normally resistant strains of mouse, leading to an IFN-γ/Th17-driven disease (Levison et al., Reference Levison, McLaughlin, Zeef, Fisher, Grencis and Pennock2010) that is controlled by a concomitant IL-10 response (Grencis et al., Reference Grencis, Humphreys and Bancroft2014). Indeed, IL-10 knock-out (KO) and IL-10R KO mice develop severe pathology in response to T. muris infection (Schopf et al., Reference Schopf, Hoffmann, Cheever, Urban and Wynn2002; Duque-Correa et al., Reference Duque-Correa, Karp, McCarthy, Forman, Goulding, Sankaranarayanan, Jenkins, Reid, Cambridge, Ballesteros Reviriego, Müller, Cantacessi, Dougan, Grencis and Berriman2019). This low-dose infection regime can be used to mimic colitis, leading to both phenotypic and transcriptional similarities to other widely used models of IBD (Levison et al., Reference Levison, McLaughlin, Zeef, Fisher, Grencis and Pennock2010; Foth et al., Reference Foth, Tsai, Reid, Bancroft, Nichol, Tracey, Holroyd, Cotton, Stanley, Zarowiecki, Liu, Huckvale, Cooper, Grencis and Berriman2014). Of 32 genes that are known to be transcriptionally different during IBD, 30 are also found to be upregulated in the CD4+CD45RB T cell transfer model of colitis (te Velde et al., Reference te Velde, de Kort, Sterrenburg, Pronk, ten Kate, Hommes and van Deventer2007). Nineteen of these 30 genes, including IFN-γ, were also found to be upregulated in chronic T. muris infection (Levison et al., Reference Levison, McLaughlin, Zeef, Fisher, Grencis and Pennock2010). Indeed, chronic T. muris infection shows a degree of similarity to all mouse models of Th1-driven colitis, both phenotypically and transcriptionally, though the degree of similarity does vary from model to model (Levison et al., Reference Levison, McLaughlin, Zeef, Fisher, Grencis and Pennock2010). Additionally, it has been shown that T. muris pathology and Crohn's disease have overlapping QTL regions – overlapping regions of DNA suggesting common genetic parameters (Levison et al., Reference Levison, Fisher, Hankinson, Zeef, Eyre, Ollier, McLaughlin, Brass, Grencis and Pennock2013). To exemplify this, the role of two different cytokines have been shown to be important in both T. muris and colitis, IL-27 and IL-13. IL-27 is a potent stimulator of Th1 responses (Pflanz et al., Reference Pflanz, Timans, Cheung, Rosales, Kanzler, Gilbert, Hibbert, Churakova, Travis, Vaisberg, Blumenschein, Mattson, Wagner, To, Zurawski, McClanahan, Gorman, Bazan, de Waal Malefyt, Rennick and Kastelein2002) and is more highly expressed in patients with IBD (Nemeth et al., Reference Nemeth, Bogdanovski, Barratt-Stopper, Paglinco, Antonioli and Rolandelli2017). However, IL-27 is also known to regulate Th17 responses and to stimulate IL-10 production and Treg generation (Awasthi et al., Reference Awasthi, Carrier, Peron, Bettelli, Kamanaka, Flavell, Kuchroo, Oukka and Weiner2007; Yoshida and Hunter, Reference Yoshida and Hunter2015). Oral delivery of IL-27 recombinant bacteria can ameliorate T cell transfer-induced colitis in mice (Hanson et al., Reference Hanson, Hixon, Li, Felber, Anver, Stewart, Janelsins, Datta, Shen, McLean and Durum2014) whilst a T. muris infection in an IL-10/IL-27 KO mouse leads to less severe pathology than seen in the IL-10 KO control due to a decreased pro-inflammatory profile (Villarino et al., Reference Villarino, Artis, Bezbradica, Miller, Saris, Joyce and Hunter2008). Additionally, WSX-1-deficient animals, that lack the functional receptor for IL-27, mount a heightened Th2 response to infection and show an accelerated expulsion of the parasite (Artis et al., Reference Artis, Villarino, Silverman, He, Thornton, Mu, Summer, Covey, Huang, Yoshida, Koretzky, Goldschmidt, Wu, de Sauvage, Miller, Saris, Scott and Hunter2004; Bancroft et al., Reference Bancroft, Humphreys, Worthington, Yoshida and Grencis2004). Despite these contrasting results, known IL-27 gene polymorphisms in IBD patients (Li et al., Reference Li, Zhang, Lee, Cho, Lee, Hahm, Choi, Yun, Chung and Chae2009; Wang et al., Reference Wang, Wang, Fan, Zhou and Zhong2014) make this cytokine an intriguing IBD therapy candidate (Andrews et al., Reference Andrews, McLean and Durum2016). In contrast, IL-13 is a Th2/Type 2 cytokine (Minty et al., Reference Minty, Chalon, Derocq, Dumont, Guillemot, Kaghad, Labit, Leplatois, Liauzun and Miloux1993) that is upregulated during an acute resolving T. muris infection (Bancroft et al., Reference Bancroft, McKenzie and Grencis1998). IL-13 is a potent suppressor of Th1 responses in humans (de Waal Malefyt et al., Reference de Waal Malefyt, Figdor, Huijbens, Mohan-Peterson, Bennett, Culpepper, Dang, Zurawski and de Vries1993; Wynn, Reference Wynn2015), although its role in IBD is complex. Crohn's disease is principally a Th1 and IFN-γ driven condition whilst ulcerative colitis is associated with increased Th2 cytokines such as IL-5 and IL-13 (Fuss et al., Reference Fuss, Neurath, Boirivant, Klein, de la Motte, Strong, Fiocchi and Strober1996, Reference Fuss, Heller, Boirivant, Leon, Yoshida, Fichtner-Feigl, Yang, Exley, Kitani, Blumberg, Mannon and Strober2004). T. muris infection in IL-10/IL-13Rα2 KO mice has been used to highlight the importance of IL-13 in controlling T. muris-induced pathology. IL-13Rα2 is the decoy receptor for IL-13 and reduces the bio-availability of IL-13 (Mentink-Kane and Wynn, Reference Mentink-Kane and Wynn2004). When infected with T. muris, IL-10/IL-13Rα2 KO mice have a decreased morbidity and mortality as compared to IL10 KO mice (Wilson et al., Reference Wilson, Ramalingam, Rivollier, Shenderov, Mentink-Kane, Madala, Cheever, Artis, Kelsall and Wynn2011) demonstrating the protective role of IL-13. In support of this, recent studies have shown that IL-13 acts to mediate recovery and repair in the gut following dextran sulphate sodium (DSS)-induced colitis, which is Th1 driven, as disease was improved in both IL-13Rα2 KO mice and in mice treated with a neutralizing IL-13Rα2 antibody (Karmele et al., Reference Karmele, Pasricha, Ramalingam, Thompson, Gieseck, Knilans, Hegen, Farmer, Jin, Kleinman, Hinds, Almeida Pereira, de Queiroz Prado, Bing, Tchistiakova, Kasaian, Wynn and Vannella2019). Additionally, transcripts for IL-13Rα2 have been found to be elevated in human IBD biopsies suggesting a protective role for IL-13 in these patients (Arijs et al., Reference Arijs, Li, Toedter, Quintens, Van Lommel, Van Steen, Leemans, De Hertogh, Lemaire, Ferrante, Schnitzler, Thorrez, Ma, Song, Marano, Van Assche, Vermeire, Geboes, Schuit, Baribaud and Rutgeerts2009, Reference Arijs, Quintens, Van Lommel, Van Steen, De Hertogh, Lemaire, Schraenen, Perrier, Van Assche, Vermeire, Geboes, Schuit and Rutgeerts2010). Similarly, patients expressing a more active variant of IL-13, with a reduced affinity to the IL-13α2 decoy receptor, had a lower risk of developing Crohn's disease (Karmele et al., Reference Karmele, Pasricha, Ramalingam, Thompson, Gieseck, Knilans, Hegen, Farmer, Jin, Kleinman, Hinds, Almeida Pereira, de Queiroz Prado, Bing, Tchistiakova, Kasaian, Wynn and Vannella2019).
Although T. muris infection can cause varied components of intestinal inflammation, the Treg response (D'Elia et al., Reference D'Elia, Behnke, Bradley and Else2009; Worthington et al., Reference Worthington, Klementowicz, Rahman, Czajkowska, Smedley, Waldmann, Sparwasser, Grencis and Travis2013; Sawant et al., Reference Sawant, Gravano, Vogel, Giacomin, Artis and Vignali2014; Duque-Correa et al., Reference Duque-Correa, Karp, McCarthy, Forman, Goulding, Sankaranarayanan, Jenkins, Reid, Cambridge, Ballesteros Reviriego, Müller, Cantacessi, Dougan, Grencis and Berriman2019) that it also initiates has been taken as a basis for a potential approach to treat IBD. The pig whipworm T. suis has been used in human trials for treatment of both Crohn's disease and ulcerative colitis with resulting remission of disease in some patients in small cohort studies (Summers et al., Reference Summers, Elliott, Urban, Thompson and Weinstock2005a, Reference Summers, Elliott, Urban, Thompson and Weinstock2005b) although no clinical improvement was seen in a larger cohort study (Schölmerich et al., Reference Schölmerich, Fellermann, Seibold, Rogler, Langhorst, Howaldt, Novacek, Petersen, Bachmann, Matthes, Hesselbarth, Teich, Wehkamp, Klaus, Ott, Dilger, Greinwald and Mueller2017). Although the exact mechanisms of action are unknown, excretory/secretory (E/S) products of T. suis on epithelial cells in vitro have been shown to elicit IL-6 and IL-10 secretion (Parthasarathy and Mansfield, Reference Parthasarathy and Mansfield2005). Additionally, when T. suis E/S products were added to bone-marrow-derived macrophages and dendritic cells, there was a reduction in secretion of pro-inflammatory cytokines and a strong enhancement of IL-10 secretion (Leroux et al., Reference Leroux, Nasr, Valanparambil, Tam, Rosa, Siciliani, Hill, Zarlenga, Jaramillo, Weinstock, Geary, Stevenson, Urban, Mitreva and Jardim2018). Remission of ulcerative colitis, following self-infection with T. trichiura, was associated with a marked elevation in IL-22 (an IL-10 family member) producing T cells which were hypothesized to promote intestinal repair by increasing goblet cell numbers and mucus production (Broadhurst et al., Reference Broadhurst, Leung, Kashyap, McCune, Mahadevan, McKerrow and Loke2010).
Barrier function in the intestine
During infection, T. muris is known to cause epithelial dysregulation in the large intestine (Artis et al., Reference Artis, Potten, Else, Finkelman and Grencis1999; Cliffe et al., Reference Cliffe, Potten, Booth and Grencis2007), a process which is also observed in human IBD (Strober et al., Reference Strober, Fuss and Mannon2007). T. muris induced TNF-α and IFN-γ production drive apoptosis within the caecal crypts of the large intestine (Artis et al., Reference Artis, Potten, Else, Finkelman and Grencis1999), which is thought to be in response to IFN-γ-induced epithelial cell hyperproliferation that also occurs (Cliffe et al., Reference Cliffe, Potten, Booth and Grencis2007) thus leading to a perturbation in intestinal homeostasis. Infection with T. trichiura, the human whipworm, may cause trichuris dysentery syndrome (Cooper et al., Reference Cooper, Bundy, MacDonald and Golden1990) in children, which is also associated with an increase in TNF-α production by mucosal macrophages (MacDonald et al., Reference MacDonald, Spencer, Murch, Choy, Venugopal, Bundy and Cooper1994). Increased intestinal apoptosis is also known to lead to a dysregulation of barrier integrity with an associated increase in epithelial permeability in IBD patients (Schulzke et al., Reference Schulzke, Bojarski, Zeissig, Heller, Gitter and Fromm2006; Mankertz and Schulzke, Reference Mankertz and Schulzke2007). During acute T. muris infection (whereby the worms are expelled before chronicity, Fig. 1), there is an accumulation of epithelial mast cells in the large intestine (Sorobetea et al., Reference Sorobetea, Holm, Henningsson, Kristiansen and Svensson-Frej2017). Mast cells produce mast cell protease-1 (MCPt-1) (Metcalfe et al., Reference Metcalfe, Baram and Mekori1997) and indeed, acute T. muris infection is associated with an increase in MCPt-1 both systemically and locally in the large intestine, which is associated with a loss of barrier integrity leading to increased epithelial permeability (Sorobetea et al., Reference Sorobetea, Holm, Henningsson, Kristiansen and Svensson-Frej2017). T. muris infection in IL-10 KO mice is known to result in marked mortality and morbidity including a loss of Paneth cells and an absence of mucus (Schopf et al., Reference Schopf, Hoffmann, Cheever, Urban and Wynn2002). Pathology in IL-10 KO and IL-10/IL-4 KO mice is also associated with bacterial outgrowth as broad-spectrum antibiotic treatment enhances survival (Schopf et al., Reference Schopf, Hoffmann, Cheever, Urban and Wynn2002). Duque-Correa et al. (Reference Duque-Correa, Karp, McCarthy, Forman, Goulding, Sankaranarayanan, Jenkins, Reid, Cambridge, Ballesteros Reviriego, Müller, Cantacessi, Dougan, Grencis and Berriman2019) also showed that IL-10 signalling had a protective effect on loss of barrier integrity leading to bacterial translocation. It is also known that T. suis E/S can affect barrier integrity by reducing the expression of tight junction proteins (Hiemstra et al., Reference Hiemstra, Klaver, Vrijland, Kringel, Andreasen, Bouma, Kraal, van Die and den Haan2014) although whether this is also a function of T. muris E/S is unknown. However, Hasnain et al. (Reference Hasnain, McGuckin, Grencis and Thornton2012) showed that adult T. muris E/S was able to degrade intestinal mucins and T. muris-induced changes in the intestinal mucus barrier have also been demonstrated that may act to increase intestinal permeability (Hasnain et al., Reference Hasnain, Wang, Ghia, Haq, Deng, Velcich, Grencis, Thornton and Khan2010, Reference Hasnain, Thornton and Grencis2011). Infection itself can lead to thickening of the glycocalyx, the glycoprotein and glycolipid covering of the intestinal epithelial cells (Linden et al., Reference Linden, Sutton, Karlsson, Korolik and McGuckin2008) likely due to the increased production of mucin proteins. However, there is also a decreased glycoprotein content within the mucosal barrier during chronic infection that may allow increased contact of the intestinal microbiota with intestinal epithelial cells (Hasnain et al., Reference Hasnain, Thornton and Grencis2011). Congruous to this, chronic T. muris infection can also alter the host intestinal microbiota (Holm et al., Reference Holm, Sorobetea, Kiilerich, Ramayo-Caldas, Estellé, Ma, Madsen, Kristiansen and Svensson-Frej2015; Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015) and it is known that a modification in the composition and function of the gut microbiota can also change intestinal permeability (Gomaa, Reference Gomaa2020).
Microbiota changes in the intestine
Changes in microbiota during a T. muris infection are evident from as early as only day 14 post-infection (p.i.). By the time that infection has reached patency (more than day 33 p.i.), there are significant changes in the composition and diversity of the microbiota (Fig. 1) (Holm et al., Reference Holm, Sorobetea, Kiilerich, Ramayo-Caldas, Estellé, Ma, Madsen, Kristiansen and Svensson-Frej2015; Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015). There was a general shift in the microbiota to a decreased number of bacteria in the Bacteroidetes phyla and an increased number of Gram-positive Lactobacillaceae. Such changes in the microbiota appear to be of benefit to the parasite and changes were transitory and required the presence of the parasite to be maintained (White et al., Reference White, Houlden, Bancroft, Hayes, Goldrick, Grencis and Roberts2018). In contrast, changes in microbiota composition in an outbred strain of mouse with a chronic T. muris infection led to an increase in bacterial invasion of the host intestinal epithelium (Schachter et al., Reference Schachter, Alvarinho de Oliveira, da Silva, de Barros Alencar, Duarte, da Silva, Ignácio and Lopes-Torres2020). Interestingly, infection-induced microbiota changes can also promote resistance to damage. In a colitis-susceptible strain of mouse (NOD2 KO), it has been established that overgrowth of Bacteroides vulgatus leads to intestinal abnormalities (Ramanan et al., Reference Ramanan, Tang, Bowcutt, Loke and Cadwell2014). However, acute infection with T. muris, that drives a Th2 response and a mucus response, led to an increase in Clostridia strains of bacteria that inhibited B. vulgatus colonization and the resulting B. vulgatus-driven abnormalities (Ramanan et al., Reference Ramanan, Bowcutt, Lee, Tang, Kurtz, Ding, Honda, Gause, Blaser, Bonneau, Lim, Loke and Cadwell2016). The microbiota of the host can also directly influence pathogenesis of T. muris as antibiotic treatment of chronically infected IL-10 KO animals, although experiencing similar pathology to control animals, had a significantly reduced mortality (Kopper et al., Reference Kopper, Patterson and Mansfield2015). Chronic infection induced changes to microflora have also been shown in T. suis infected pigs (Li et al., Reference Li, Wu, Li, Navarro, Couch, Hill and Urban2012) although there is contrasting evidence as to whether the human whipworm also drives microflora changes (Cooper et al., Reference Cooper, Walker, Reyes, Chico, Salter, Vaca and Parkhill2013; Ramanan et al., Reference Ramanan, Bowcutt, Lee, Tang, Kurtz, Ding, Honda, Gause, Blaser, Bonneau, Lim, Loke and Cadwell2016).
Trichuris effects distal to the site of infection
Despite its intestinal epithelial location, the effects of T. muris infection are not only restricted to the site of infection. Chronic T. muris infection can modulate responses to chemical skin sensitizers applied to the ear of the mouse. Suppression of local cellular/cytokine Th1/pro-inflammatory responses and ear pathology were observed when using a Th1-promoting compound [2,4-dinitrochlorobenzene (DNCB)] although no depression in IL-13, or ear swelling was noted after sensitizing with the Th2-promoting compound trimellitic anhydride (TMA). Interestingly, the suppression of pathology after DNCB treatment was associated with a reduction in egress of dendritic cells (DCs) from the skin coincident with elevated IL-10 production and a slight increase in CD4+FoxP3+ cells in the draining lymph node (Grencis et al., Reference Grencis, Humphreys and Bancroft2014). Movement of DCs from the skin to the draining lymph node has been shown to be dependent on local proinflammatory cytokines which can be inhibited by IL-10 production (Cumberbatch et al., Reference Cumberbatch, Dearman, Griffiths and Kimber2000).
T. muris effects in the lungs
Chronic T. muris infection which drives a strong Th1 response in the intestine, has also been shown to drive the production of IFN-γ (by Th1 cells) and IL-10 (myeloid cells) in the lung of the host (Fig. 1), and so has the potential to suppress the development of Type-2-driven airway inflammation (Chenery et al., Reference Chenery, Antignano, Burrows, Scheer, Perona-Wright and Zaph2016). The increased Th1 type response in the lung was able to reduce the lung response to both papain and house-dust mite, together with a reduced eosinophil infiltration and reduced lung mucus production. IL-17 is another cytokine known to be increased in complex asthma and may contribute to disease progression (Doe et al., Reference Doe, Bafadhel, Siddiqui, Desai, Mistry, Rugman, McCormick, Woods, May, Sleeman, Anderson and Brightling2010): additionally, IL-17 is critical for neutrophil expansion and remodelling of lung tissue and may contribute to disease progression in other chronic respiratory conditions (Gurczynski and Moore, Reference Gurczynski and Moore2018). A high-dose infection of T. muris, that induces a Th2 response (Fig. 1), can promote a mixed IL-17 and Th2-type immunity to the parasite (Wilson et al., Reference Wilson, Ramalingam, Rivollier, Shenderov, Mentink-Kane, Madala, Cheever, Artis, Kelsall and Wynn2011). Induction of Th2 cytokines can also be seen in the host lung following infection with a high dose of T. muris, however, this is dependent on IL-17 production and is ablated in an IL-17 KO animal (Ajendra et al., Reference Ajendra, Chenery, Parkinson, Chan, Pearson, Colombo, Boon, Grencis, Sutherland and Allen2020). Interestingly, this IL-17-dependent suppression of IFN-γ, which allowed the promotion of type-2 immune responses, was only apparent in the host lung and was not seen in the intestine. Additionally, a secreted product from T. muris, p43, is able to bind to IL-13 in vitro and in vivo (Bancroft et al., Reference Bancroft, Levy, Jowitt, Hayes, Thompson, McKenzie, Ball, Dubaissi, France, Bellina, Sharpe, Mironov, Brown, Cook, MacDonald, Thornton and Grencis2019). When given to mice intranasally with IL-13, p43 reduced the percentage of RELM-β positive interstitial lung macrophages as compared to mice treated with IL-13 only. The effects of p43 are further reviewed in this special issue by Bancroft & Grencis. By-stander effects of Trichuris infection in the lung are also seen with other species of Trichuris. T. suis ova treatment in a grass-pollen allergy clinical trial increased Th2 and IL-10 production in patients although this did not affect allergen-specific cytokine responses (Bourke et al., Reference Bourke, Mutapi, Nausch, Photiou, Poulsen, Kristensen, Arnved, Rønborg, Roepstorff, Thamsborg, Kapel, Melbye and Bager2012). Interestingly, treatment of ovalbumin-sensitized mice with T. suis larval E/S proteins suppressed airway hyperreactivity and bronchiolar inflammation, partially mediated by E/S-induced IL-10 secretion (Ebner et al., Reference Ebner, Hepworth, Rausch, Janek, Niewienda, Kühl, Henklein, Lucius, Hamelmann and Hartmann2014). Whether T. trichiura has similar abilities to modulate inflammation is uncertain and there are conflicting results in the literature (Rodrigues et al., Reference Rodrigues, Newcombe, Cunha, Alcantara-Neves, Genser, Cruz, Simoes, Fiaccone, Amorim, Cooper and Barreto2008; Alcântara-Neves et al., Reference Alcântara-Neves, Badaró, dos Santos, Pontes-de-Carvalho and Barreto2010; Gonçales et al., Reference Gonçales, Nobrega, Nascimento, Lorena, Peixoto, Costa, Barbosa, Solé, Sarinho and Souza2020).
T. muris cerebrovascular and neurodegenerative disease
It is well established that infection and systemic inflammation are risk factors for ischaemic brain damage (stroke) and can also affect the progression of some neurodegenerative disorders (He et al., Reference He, Zhang, Pan, Tai, Liang and Shi2020).
Using transient middle cerebral artery occlusion as a model of stroke it was shown that a chronic low-dose T. muris infection, which drives a Th1 response (Fig. 1), dramatically exacerbated brain damage caused by experimental stroke (Dénes et al., Reference Dénes, Humphreys, Lane, Grencis and Rothwell2010). Infection led to an increase in pro-inflammatory mediators in the brain and surrounding tissue together with an altered Treg response. Infected mice had elevated Th1-associated cytokines and chemokines after cerebral artery occlusion however, only CCL5 (RANTES) stayed significantly increased after 48 hours post-stroke. Anti-RANTES treatment prevented the infection-driven exacerbation of stroke-induced damage. Analysis of matrix metallopeptidase 9 expression in the brain showed elevated levels after stroke and infection compared to stroke alone indicating augmented vascular injury and blood−brain barrier damage in chronically infected animals. Interestingly, an acute, resolving T. muris infection driving a Th2 response had no effect on infarct size demonstrating that it was the Th1 milieu driven by the parasite that was detrimental rather than the parasite itself (Dénes et al., Reference Dénes, Humphreys, Lane, Grencis and Rothwell2010). The detrimental effects of infection are also very much dependent on age as infarct size was found to be significantly increased in chronically infected aged mice as compared to chronically infected young mice (Dhungana et al., Reference Dhungana, Malm, Denes, Valonen, Wojciechowski, Magga, Savchenko, Humphreys, Grencis, Rothwell and Koistinaho2013). Older mice experienced an increased neutrophil recruitment and upregulation of Th1 cytokines as compared to the younger mice leading to the increased pathology seen.
As well as stroke, it has also been demonstrated that chronic T. muris infection can accelerate the onset of experimental clinical prion disease – a chronic, neurodegenerative disease caused by infectious proteins (Donaldson et al., Reference Donaldson, Bradford, Else and Mabbott2020). Mice were infected with a chronic T. muris infection after receiving prions, timed so that the peak of parasite-driven inflammation would coincide with known pre-clinical phases of the prion infection. T. muris infected mice had a reduced survival time which correlated with increased pro-inflammatory cytokines in the sera and increased numbers of CD8+ cells in the brain (Donaldson et al., Reference Donaldson, Bradford, Else and Mabbott2020). T. muris infection can also exacerbate neuroinflammation in models of Alzheimer's disease, a chronic neurodegenerative condition (Querfurth and LaFerla, Reference Querfurth and LaFerla2010; Montacute et al., Reference Montacute, Foley, Forman, Else, Cruickshank and Allan2017). Infection in the Alzheimer's mouse model (3xTg-AD) led to increased levels of inflammation in the brain with increased microglia activation. Interestingly, these transgenic animals were also unable to fully expel a high-dose infection, which is normally acute and resolving (Fig. 1), together with increased Th1 cytokine levels in response to infection in the lymph node draining the large intestine (Montacute et al., Reference Montacute, Foley, Forman, Else, Cruickshank and Allan2017). Although not addressed in any T. muris infection model, T. suis E/S effects in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis (MS), have been assessed (Kuijk et al., Reference Kuijk, Klaver, Kooij, van der Pol, Heijnen, Bruijns, Kringel, Pinelli, Kraal, de Vries, Dijkstra, Bouma and van Die2012; Hansen et al., Reference Hansen, Hasseldam, Bacher, Thamsborg, Johansen and Kringel2017). Intraperitoneal administration of T. suis E/S before disease onset significantly decreased disease severity and markedly reduced systemic Th1 and Th17 responses (Hansen et al., Reference Hansen, Hasseldam, Bacher, Thamsborg, Johansen and Kringel2017). However, T. suis ova therapy in MS clinical trials have had mixed effects (Voldsgaard et al., Reference Voldsgaard, Bager, Garde, Åkeson, Leffers, Madsen, Kapel, Roepstorff, Thamsborg, Melbye, Siebner, Søndergaard, Sellebjerg and Sørensen2015; Fleming et al., Reference Fleming, Hernandez, Hartman, Maksimovic, Nace, Lawler, Risa, Cook, Agni, Reichelderfer, Luzzio, Rolak, Field and Fabry2019; Yordanova et al., Reference Yordanova, Ebner, Schulz, Steinfelder, Rosche, Bolze, Paul, Mei and Hartmann2021).
Trichuris and coinfections
Surprisingly little work has been carried on coinfections of T. muris and viral or bacterial infections though some work has been done with Mycobacteria and Streptococcus. Immunity to Mycobacterium bovis (M. bovis) infection has been shown to be negatively influenced by a T. muris coinfection. A high-dose T. muris infection, which promotes a Th2 response, down-regulated pulmonary Th1 and Treg cell responses to the bacteria (Fig. 1) (Nel et al., Reference Nel, du Plessis, Kleynhans, Loxton, van Helden and Walzl2014) although this had no effect on bacterial proliferation and dissemination. However, T. muris E/S-treated human monocyte-derived macrophages prior to exposure to M. tuberculosis led to an M2-type polarization with reduced macrophage phagosome maturation and a resulting increased bacterial burden (Aira et al., Reference Aira, Andersson, Singh, McKay and Blomgran2017). In a T. muris-Streptococcus pneumoniae coinfection model, nematode infection was associated with an increased carriage of S. pneumoniae, though this did not reach significance, with a significant increase in dissemination of the bacteria to the lungs (Law et al., Reference Law, Shears, Lopez Rodas, Grencis, Cooper, Neill and Kadioglu2021). Anthelmintic treatment led to a smaller, though not significant, load of bacteria. This trend for a higher carriage of bacteria when coinfected with Trichuris was similarly seen in children harbouring T. trichiura (Law et al., Reference Law, Shears, Lopez Rodas, Grencis, Cooper, Neill and Kadioglu2021).
Protozoan infections such as Plasmodium berghei, Trypanosoma brucei and Babesia microti and B. hylomysci will all delay the expulsion of a high dose of T. muris infection, particularly at times of high parasitaemia suggesting that at least acute T. muris infections do not exert strong immunomodulatory effects on these co-infections (Phillips and Wakelin, Reference Phillips and Wakelin1974; Phillips et al., Reference Phillips, Selby and Wakelin1974).
More data are available on the effect of T. muris infection on other helminth infections. Experimental infection of Nematospiroides dubius [Heligmosomoides polygyrus (bakerii)], which resides in the small intestine, delayed expulsion of a high dose T. muris infection and enhanced survival of a trickled T. muris infection (Behnke et al., Reference Behnke, Ali and Jenkins1984). The lung, like the gut, is a mucosal surface and many helminth parasites have evolved a migratory phase through the lungs in their life cycle (Craig and Scott, Reference Craig and Scott2014). Cross-talk between the lung and intestinal mucosal surfaces in terms of host immunity is particularly evident during helminth co-infections. Nippostrongylus brasiliensis is a rodent small intestinal dwelling parasite that migrates through the host lung before reaching maturity (Bouchery et al., Reference Bouchery, Volpe, Shah, Lebon, Filbey, LeGros and Harris2017). Intestinal infection with a high dose of T. muris, that promotes a Th2 response and is expelled by the host (Fig. 1), reduced the number of N. brasiliensis larvae found in the lung at d2 post-infection (Filbey et al., Reference Filbey, Camberis, Chandler, Turner, Kettle, Eichenberger, Giacomin and Le Gros2019). Interestingly, mice that had been given a trickle infection of T. muris (initially driving a Th1 response and then a protective Th2 response) and then a N. brasiliensis infection, after the switch to a Th2 dominated response, had an equivalent number of larvae in the lung at d3 post-infection as WT mice (Glover et al., Reference Glover, Colombo, Thornton and Grencis2019). This suggests either a resolving delay in N. brasiliensis migration in the lung as equivalent numbers of adults were found in the intestine (Glover et al., Reference Glover, Colombo, Thornton and Grencis2019) or a qualitative difference in the Th2 response initiated by a high dose as compared to a trickle infection.
T. muris-induced alteration in the lung cytokine expression has also been demonstrated in co-infection with Schistosoma mansoni (Bickle et al., Reference Bickle, Solum and Helmby2008). S. mansoni is a trematode that causes chronic infection in mice, causing pathology in the lungs as it migrates (Boros, Reference Boros1989). Chronic infection with T. muris led to a reduced trapping of larvae during their skin-to-lung migration associated with an altered lung cytokine expression. Interestingly, co-infected lungs had a lower expression of IFN-γ despite the Trichuris-driven Th1 response, and it was actually an IL-10-dominated response that appeared to limit antilarval schistosomula immunity (Bickle et al., Reference Bickle, Solum and Helmby2008) and allowed progression of the parasite to the portal system with resulting increased egg burden and pathology in co-infected mice. Conversely, a chronic T. muris infection can be resolved by a Schistosome coinfection due to the S. mansoni egg-induced Th2 response (Curry et al., Reference Curry, Else, Jones, Bancroft, Grencis and Dunne1995). Additionally, S. mansoni and T. muris coinfected mice had significantly higher burden of adult Schistosome worms and eggs in the liver (Bickle et al., Reference Bickle, Solum and Helmby2008) thus demonstrating that contrasting effects that the infections can have on one another.
Trichuris and neoplasia
Cancer is a leading cause of death in high-income countries and incidences are increasing in low-income countries. There exists a strong link between inflammation and cancer with chronic infection and the long-term exposure to inflammatory stimuli heightening the risk of neoplastic change (Wang and Wang, Reference Wang and Wang2007).
Chronic T. muris infection at day 80 p.i. in a wild-type mouse led to the development of neoplastic change that was similar to that seen in mice that had been treated with the carcinogen azoxymethane (Hayes et al., Reference Hayes, Cliffe, Bancroft, Forman, Thompson, Booth and Grencis2017). Intestinal crypt structure was altered alongside increased incidence of pre-adenomas which were more pronounced (in the case of aberrant crypt foci) in the infected mice as compared to the chemically treated mice. Even though T. muris infection can lead to increased epithelial proliferation and apoptosis in the intestine (Artis et al., Reference Artis, Potten, Else, Finkelman and Grencis1999; Cliffe et al., Reference Cliffe, Potten, Booth and Grencis2007), both of which can lead to tumour formation (Evan and Vousden, Reference Evan and Vousden2001) these intestinal changes were only apparent in the caecum, the parasite niche, rather than throughout the small intestinal tract where neoplastic change was mostly observed (Hayes et al., Reference Hayes, Cliffe, Bancroft, Forman, Thompson, Booth and Grencis2017). Neoplastic change was seen in chronically infected animals even before the peak of parasite-specific cytokine responses was evident in the draining lymph node, although greater significant differences were seen as infection progressed. Infection generated a Th1-predominant response in these animals, however, this was not associated with a reduced neoplasia as might have been expected (Wang et al., Reference Wang, Wang, Song, Chu and Qu2015).
The APCmin/+ tumour model in the mouse develops spontaneous adenomas throughout the GI tract (Moser et al., Reference Moser, Pitot and Dove1990). Chronic infection of APCmin/+ mice with T. muris led to a significant increase in new tumour formation throughout the intestine and not just an increase in tumour size. Blockade of the CD25+ Treg response abrogated this heightened tumour formation demonstrating the role of the T. muris-induced Tregs in regulating the anti-tumour response in these animals (Hayes et al., Reference Hayes, Cliffe, Bancroft, Forman, Thompson, Booth and Grencis2017). Tregs have also been characterized within tumour microenvironments that can induce tumour-specific immune tolerance (Wang and Wang, Reference Wang and Wang2007). Clonal expansion of tumour Tregs is thought to occur both locally and systemically and a high proportion of Tregs with the tumour micro-environment is correlative with poor prognosis in many cancer types suggestive of the suppressive role of Tregs on anti-tumour immunity (Mougiakakos, Reference Mougiakakos2011; Fridman et al., Reference Fridman, Pagès, Sautès-Fridman and Galon2012; Ahmadzadeh et al., Reference Ahmadzadeh, Pasetto, Jia, Deniger, Stevanović, Robbins and Rosenberg2019). Interestingly T. suis E/S proteins are capable of stimulating the secretion of IL-10 from macrophages though failed to induce CD25+Foxp3+ T cells unlike T. muris E/S which was able to do this (D'Elia et al., Reference D'Elia, Behnke, Bradley and Else2009; Leroux et al., Reference Leroux, Nasr, Valanparambil, Tam, Rosa, Siciliani, Hill, Zarlenga, Jaramillo, Weinstock, Geary, Stevenson, Urban, Mitreva and Jardim2018). Additionally, increased mucosal T cell activation production of IL-10, TGF-β and FoxP3 were found in the colon of an individual with ulcerative colitis who self-infected with T. trichiura (Dige et al., Reference Dige, Rasmussen, Nejsum, Hagemann-Madsen, Williams, Agnholt, Dahlerup and Hvas2017). Tregs are known to play a role in both pathology and immunity early on following chronic T. muris infection as are TGF-β and IL-10 (D'Elia et al., Reference D'Elia, Behnke, Bradley and Else2009; Worthington et al., Reference Worthington, Klementowicz, Rahman, Czajkowska, Smedley, Waldmann, Sparwasser, Grencis and Travis2013; Sawant et al., Reference Sawant, Gravano, Vogel, Giacomin, Artis and Vignali2014; Duque-Correa et al., Reference Duque-Correa, Karp, McCarthy, Forman, Goulding, Sankaranarayanan, Jenkins, Reid, Cambridge, Ballesteros Reviriego, Müller, Cantacessi, Dougan, Grencis and Berriman2019). It is noteworthy however, that low-dose chronic infection with T. muris is associated with a depression in Foxp3+CD4+T cells in the caecum and colon (Holm et al., Reference Holm, Sorobetea, Kiilerich, Ramayo-Caldas, Estellé, Ma, Madsen, Kristiansen and Svensson-Frej2015; Houlden et al., Reference Houlden, Hayes, Bancroft, Worthington, Wang, Grencis and Roberts2015). Taken together these data suggest that distinct populations of CD4+ T cells are involved in regulating tumours at sites away from the parasite niche.
IL-10 and TGF-β are not the only regulatory cytokines associated with a T. muris infection and cancer. IL-35 is an immune-suppressive cytokine which belongs to the IL-12 cytokine family and can also act to regulate Th1 immunity (Collison et al., Reference Collison, Workman, Kuo, Boyd, Wang, Vignali, Cross, Sehy, Blumberg and Vignali2007). Chronic T. muris infection can drive an inducible cell type (iT(R)35 cells) that exert regulatory effects via IL-35 and are Foxp3 independent (Collison et al., Reference Collison, Chaturvedi, Henderson, Giacomin, Guy, Bankoti, Finkelstein, Forbes, Workman, Brown, Rehg, Jones, Ni, Artis, Turk and Vignali2010). In a melanoma model of cancer, these T. muris-induced cells can be found within the tumour micro-environment (in the skin) and contributed to tumour progression by again regulating the ongoing anti-tumour responses (Collison et al., Reference Collison, Chaturvedi, Henderson, Giacomin, Guy, Bankoti, Finkelstein, Forbes, Workman, Brown, Rehg, Jones, Ni, Artis, Turk and Vignali2010). In addition, IL-31 is a Th2 T cell cytokine that can suppress type 2 immune responses (Dillon et al., Reference Dillon, Sprecher, Hammond, Bilsborough, Rosenfeld-Franklin, Presnell, Haugen, Maurer, Harder, Johnston, Bort, Mudri, Kuijper, Bukowski, Shea, Dong, Dasovich, Grant, Lockwood, Levin, LeCiel, Waggie, Day, Topouzis, Kramer, Kuestner, Chen, Foster, Parrish-Novak and Gross2004). IL-31 and IL31R play a regulatory role in T. muris infection with an induced production of this cytokine in the intestine following infection (Perrigoue et al., Reference Perrigoue, Zaph, Guild, Du and Artis2009). Additionally, infection of IL31R KO mice led to a heightened Th2 cytokine response and enhanced goblet cell hyperplasia with a resulting accelerated expulsion of worms. As this cytokine has also been implicated in cancer progression, it is likely that T. muris induced IL-31 production may also enhance tumour progression in a manner similar to IL-35 (He et al., Reference He, Zhang, Pan, Tai, Liang and Shi2020).
Conclusion
T. muris is an intestinal dwelling nematode parasite that can have far-reaching consequences in the host (Fig. 1). Within the intestine itself, chronic T. muris in susceptible strains can have pathological consequences that show a degree of similarity to symptoms of IBD. Indeed, several genes upregulated during a chronic T. muris infection are also found to be upregulated in IBD patients. Paradoxically, T. muris infections can also help modulate IBD symptoms and pathologies due to the parasite-specific Treg response driven by infection. T. muris also drives microbiota changes in the host, beneficial to its survival, that have consequences for the host due to the impact that these changes can have on mucus constituents and intestinal permeability. Distal from the site of infection, T. muris infections can have an impact on immune responses to chemical sensitizers in the ear. In this case, a chronic T. muris driven IL-10 production preventing the egress of DCs from the ear. Chronic T. muris infection can also modulate immune responses in the lung to airway allergens which was also associated with an increased IL-10 response. T. muris infection can also influence immune responses in the brain and it has been demonstrated that an on-going T. muris-driven Th1 response will worsen the damage caused by experimental stroke, a process driven by an elevated and sustained RANTES production. Additionally, T. muris can have an effect on other brain inflammations with papers reporting changes in prion diseases and Alzheimer's progression. Although relatively little work has addressed the effects of T. muris on other parasite, viral and microbial infections, altered immunity to mycobacteria, pneumococcus, N. brasiliensis, H. bakerii and S. mansoni have been reported. Finally, effects of T. muris infection on cancer progression establish that the T. muris-driven Treg response plays an important role in inhibiting host immunity to adenoma progression in the intestine leading to development of more tumours. Additionally, two other regulatory cytokines, IL-35 and IL-31, induced by T. muris infection are able to modulate tumour immunity. In light of this, the importance of T. muris infections on other diseases and other body systems is profound and warrants further research and investigation, especially considering the widespread nature of this parasite in the human population.
Author contributions
KSH conducted the literature search, designed and wrote the review and figure. RKG provided feedback and comments on the manuscript.
Financial support
The work in RKG's laboratory is funded by Wellcome Trust investigator award (Z10661/Z/18/Z). The Wellcome Trust Centre for Cell Matrix Research, University of Manchester is also supported by centre funding from the Wellcome Trust (088785/Z/09/Z).
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable