Published online by Cambridge University Press: 10 February 2022
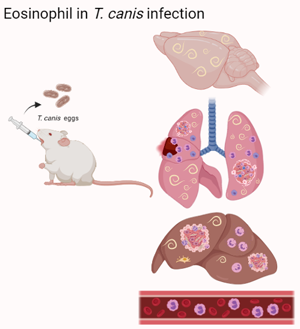
An increase in peripheral blood eosinophils in helminth infections is expected, and these cells are known to promote immunity against these parasites. However, studies have suggested that in some specific helminths, eosinophils may promote the needs and longevity of these parasites, and their role in these infections remains undefined, including in Toxocara canis infection. Thus, this study aimed to investigate the role of eosinophils in the context of larval migration of T. canis and the immunopathological aspects of infection. For this, we used wild-type mice and mice genetically deficient for the transcription factor GATA-binding factor 1 (GATA1−/−), infected with 1000 eggs of T. canis. At 0, 3, 14 and 63 days post-infection, parasite load, tissue cytokine production, leucocyte profile, bronchoalveolar lavage cells and histopathological analyses were carried out. Collectively, our results demonstrate that the presence of eosinophils mediates susceptibility to T. canis, inducing leucocytosis and the formation of granulomas, increasing the pulmonary and cerebral parasite load, and reducing the number of neutrophils, which may be necessary to control the infection.