Published online by Cambridge University Press: 05 October 2021
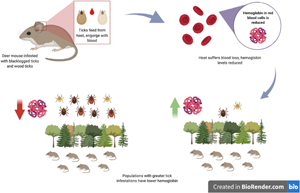
Deer mice (Peromyscus maniculatus) are hosts to ixodid ticks as well as the associated tick-borne pathogens they can spread. As the ranges of black-legged ticks (Ixodes scapularis) and American dog ticks (Dermacentor variabilis) expand northwards, naïve host populations of deer mice are likely to become infested by ticks and experience the physiological effects that ticks can have on them via blood-feeding. The prevalence of these haematophagous ticks can affect the haemoglobin levels of the mice they infest. Haemoglobin levels were compared and analysed in deer mice populations at three different sites with varying tick exposure. These results suggested that without confounding effects, the abundance of black-legged and American dog ticks on individual mice had a significant negative effect on the hosts' haemoglobin levels, but only in an area with high tick infestation. This was seen across the average haemoglobin levels between populations, where there was a significant difference between the source population with the longest established tick populations and the source population where neither black-legged nor American dog ticks were prevalent. As the ticks' ranges expand and they become more abundant, it is important to understand how their prevalence and intensity can alter host physiology, potentially affecting their own range expansion and the spread of the diseases they may carry.