Published online by Cambridge University Press: 12 August 2021
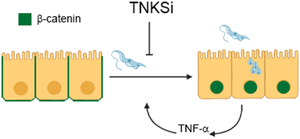
Chagas disease is a potentially life-threatening protozoan infection affecting around 8 million people, for which only chemotherapies with limited efficacy and severe adverse secondary effects are available. The aetiological agent, Trypanosoma cruzi, displays varied cell invading tactics and triggers different host cell signals, including the Wnt/β-catenin pathway. Poly(ADP-ribose) (PAR) can be synthetized by certain members of the poly(ADP-ribose) polymerase (PARP) family: PARP-1/-2 and Tankyrases-1/2 (TNKS). PAR homoeostasis participates in the host cell response to T. cruzi infection and TNKS are involved in Wnt signalling, among other pathways. Therefore, we hypothesized that TNKS inhibitors (TNKSi) could hamper T. cruzi infection. We showed that five TNKSi (FLALL9, MN64, XAV939, G007LK and OULL9) diminished T. cruzi infection of Vero cells. As most TNKSi did not affect the viability of axenically cultivated parasites, our results suggested that TNKSi were interfering with parasite–host cell signalling. Infection by T. cruzi induced nuclear translocation of β-catenin, as well as upregulation of TNF-α expression and secretion. These changes were hampered by TNKSi. Further signals should be monitored in this model and in vivo. As a TNKSi has entered cancer clinical trials with promising results, our findings encourage further studies aiming at drug repurposing strategies.