INTRODUCTION
Soil-transmitted helminthiasis (STH) is amongst the most prevalent parasitic diseases in sub-Saharan Africa, being present in almost every socio-economically depressed community (de Silva et al. Reference De Silva, Chan and Bundy1997, Reference De Silva, Brooker, Hotez, Montresor, Engels and Savioli2003; Crompton, Reference Crompton1999). In environments where poor sanitation and hygiene abound, STH can have a major detrimental impact upon the general well-being of infants (<1 year) and pre-schoolchildren (<5 years) (Crompton, Reference Crompton2001; Stoltzfus et al. Reference Stoltzfus, Chway, Montresor, Tielsch, Jape, Albonico and Savioli2004). In line with the Millennium Development Goals (MDGs), large-scale control of STH is set firmly within an agreed international health agenda (Savioli et al. Reference Savioli, Stansfield, Bundy, Mitchell, Bhatia, Engels, Montresor, Neira and Shein2002, Reference Savioli, Engels and Endo2005) and, following WHO guidelines, is implemented within a framework of preventive chemotherapy with orally administered anthelminthics (Albonico et al. Reference Albonico, Montresor, Crompton and Savioli2006, Reference Albonico, Allen, Chitsulo, Engels, Gabrielli and Savioli2008; Crompton and Savioli, Reference Crompton and Savioli2007).
STH is of public health importance on each of the two islands of Zanzibar, Unguja and Pemba (Albonico et al. Reference Albonico, Chwaya, Montresor, Stolfzus, Tielsch, Alawi and Savioli1997, Reference Albonico, Montresor, Crompton and Savioli2006), and in 1994 the Zanzibarian Ministry of Health and Social Welfare (MoHSW) established the Helminth Control Programme (HCP) (Renganathan et al. Reference Renganathan, Ercole, Albonico, de Gregorio, Alawi, Kisumku and Savioli1995). On Unguja, the HCP operates from the Helminth Control Laboratory (HCLU) which is an island-wide out-patient referral centre dispensing de-worming medications such as albendazole (ALB), mebendazole (MEB) and praziquantel (PZQ) and, when sufficient donor funds are available, acts as an implementation unit providing the necessary infrastructure and resources for mass drug administration (MDA) campaigns targeted mainly towards the schoolaged child (Stothard et al. Reference Stothard, Loxton, Rollinson, Mgeni, Khamis, Ameri, Ramsan and Savioli2000, Reference Stothard, Mgeni, Khamis, Seto, Ramsan, Hubbard, Kristensen and Rollinson2002a, Reference Stothard, Mgeni, Khamis, Seto, Ramsan and Rollinsonb). In 1994 HCLU conducted a comprehensive parasitological baseline assessment of STH within Ungujan primary schools and while MDA continued over the next decade, albeit sporadically, further parasitological monitoring was only possible from 2003 when additional financial support became available from the 4-year Piga vita Kichocho (Kick out Kichocho) programme. Kick out Kichocho is a partnership between the MoHSW and Natural History Museum, UK with additional support from the African Development Bank, Tanzania and the Schistosomiasis Control Initiative, UK. The programme focuses upon combined control of urinary schistosomiasis and STH with annual MDA of PZQ and ALB in primary schools, targeting some 130 000 school children (Southgate et al. Reference Southgate, Rollinson, Tchuente and Hagan2005; Stothard et al. Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006). In addition, 4 annual parasitological surveys have been completed in 24 sentinel primary schools, following approximately 4000 children to track the impact of treatment upon urinary schistosomiasis and STH (French et al. Reference French, Rollinson, Basanez, Mgeni, Khamis and Stothard2007).
The present situation of STH within Ungujan infants and pre-schoolaged children is, however, much less clear. While mother and child health (MCH) clinics have been distributing vitamin A, childhood vaccinations and benzimidazoles (ALB or MEB) twice a year from 2004, there has been insufficient surveillance of STH within this mother and child group as MCH clinics do not undertake parasitological monitoring. With the realization that infants and pre-schoolchildren could also be at significant risk of schistosomiasis (Stothard and Gabrielli, Reference Stothard and Gabrielli2007), a pilot epidemiological survey of urinary schistosomiasis and STH was undertaken in January 2006 targeting this mother and child group. Examining a total of 120 mothers and 155 children across 4 northern Ungujan villages (Sousa-Figueiredo et al. Reference Sousa-Figueiredo, Basanez, Mgeni, Khamis, Rollinson and Stothard2008), the survey confirmed that urinary schistosomiasis was rare within infants and pre-schoolchildren (<1%) owing to their lack of environmental water contact but uncovered surprisingly high levels of STH [(in children, ascariasis 32·1% and trichuriasis 38·4%), (in mothers, ascariasis 18·7% and trichuriasis 24·2%)]. By contrast, prevalence of hookworm was much lower at only 0·9% and 5·5% in children and mothers, respectively (Sousa-Figueiredo et al. Reference Sousa-Figueiredo, Basanez, Mgeni, Khamis, Rollinson and Stothard2008). The comparatively low prevalence of hookworm, which historically has been at least 30-fold higher in the schoolaged child (HCLU, unpublished findings), raised an immediate concern that benzimidazoles were performing less well at containing ascariasis and trichuriasis. Moreover, the study drew attention to the general absence of relevant island-wide epidemiological information on STH which would be important for assessing the current and future impact of MCH clinics on de-worming.
In this paper we report of the findings of a more inclusive island-wide parasitological survey of STH in infants and pre-schoolchildren and their mothers, supplemented with case-history questionnaires to identify local infection risk factors. In seeking better documentation of Ascaris adult worm burdens, adult worms were expelled and collected.
MATERIALS AND METHODS
Study area and population
The study was conducted in 10 villages on Unguja Island representative of urban, semi-urban and rural environments. After liaison with the local Shehia (the elected community leader) mothers and their children aged between 6 months and 5 years were invited to attend a walk-in mobile clinic. In accordance with WHO sample size recommendations of 30 individuals per site (Montresor et al. Reference Montresor, Crompton, Bundy, Hall and Savioli1998, Reference Montresor, Crompton, Gyorkos and Savioli2002) an initial target enrolment of 50 mother and child pairs at each study village was set to cater for drop-out (e.g. absence at clinic) and non-compliance (e.g. failure to produce stool) during the ensuing study period.
Prevalence survey and questionnaire
The surveys were scheduled to precede immediately before the summer 2006 mother and child clinics when de-worming would take place. Two trained members of the HCLU team first explained the aims of the survey to attending mothers. Stool containers were distributed and labelled separately for mother and child, with instructions for their use. Collection of specimens took place the following day. After obtaining informed consent, the name, age and sex of each mother and child pair were noted and each mother was asked a suite of 11 yes/no questions in Kiswahili from a standardized case-history questionnaire of the following format. (1) Do you know what STH is? (2) Does your household have a latrine? (3) Have you recently accessed local health services? (4) Have you ever taken STH treatment? (5) Has your child ever taken STH treatment? (6) Has your child had a Vitamin A supplement? (7) Has your child had immunizations? (8) Does your child usually wear shoes/sandals? (9) Does your child often play on the ground? (10) Has your child ever passed blood in stool? (11) Has your child ever passed worms in stool?
Stool specimens were transported to the HCLU laboratory for visual inspection of stool consistency and presence of blood, after which a single Kato-Katz thick smear (41·7 mg) was prepared (Montresor et al. Reference Montresor, Crompton, Bundy, Hall and Savioli1998). Eggs of all STH species were counted by inspection at 100× microscopy and expressed as a tally of eggs per gramme (EPG) (Montresor et al. Reference Montresor, Crompton, Bundy, Hall and Savioli1998). To ensure consistency of egg counts, slides were read by the same two technicians for each study village. As a later quality control, a random sample of 10% was read by one of the authors to confirm original estimations, if the quality control showed a >10% difference, which was rare, the slide was re-read.
Expulsion of Ascaris and measurements
On the day following STH diagnosis, a total of 24 children from 4 villages were selected upon the basis of having either a moderate or heavy intensity of Ascaris infection. These children then received a single treatment of Combantrin®, an oral formulation of pyrantel pamoate made by Pfizer, UK. Combantrin® (10 mg/kg) was administered in half or quarter-tablet divisions or in 2·5 ml or 5 ml syrup aliquots. All treatments were observed and none was rejected. Each mother received a plastic bag with sealable collar for collection of expelled worms during the ensuing 24-h period. At the first study site of Kandwi village, a second treatment with Combantrin® was administered to assess if any further worms were expelled. As no additional worms were retrieved, a single treatment with Combantrin® and 24-h follow-up was conducted at other villages to standardize study conditions.
Upon collection, adult worms were counted and washed in normal saline to remove faecal debris. After blotting worms dry, total worm length and worm mass were measured to the nearest 0·1 cm and 0·01 g, respectively. The sex of each worm was determined at 100× magnification by the presence or absence of eggs (Hall et al. Reference Hall, Anwar, Tomkins and Rahman1999).
Data-handling and analysis
Data were first tabulated with Microsoft Excel® and summaries were made of intensities of infections using both arithmetic mean egg counts of positive individuals alone and geometric mean egg counts of all surveyed individuals. Infections were categorised as ‘light’, ‘moderate’ or ‘heavy’ according to WHO classifications (Montresor et al. Reference Montresor, Crompton, Bundy, Hall and Savioli1998). The distribution of parasites in hosts can be described by a negative binomial distribution, the shape of which is characterized by the over-dispersion parameter (k), as k trends towards zero the parasite population becomes progressively more aggregated, or clumped, within the sampled population. Using EPG data for each STH species and adult Ascaris worm burdens, k was estimated by using maximum likelihood (Martin et al. Reference Martin, Keymer, Isherwood and Wainwright1983) and goodness of fit was tested using chi-squared test (Williams and Dye, Reference Williams and Dye1994).
The importance of questionnaire responses, scored binomially, as risk factors for STH was assessed using summary odds ratios (OR) across mothers and children, mothers alone and children alone calculated using R statistical package v2.6 (© R Foundation, Vienna, Austria). Since mothers and children lived in different villages, the possibility of intra-correlation in the data was assumed, therefore, univariate and multivariate analyses were carried out using a multilevel logistic regression model controlling for random effects at the village level. A final, most parsimonious, model was established using Akaike information criterion (AIC) (Akaike, Reference Akaike, Petrov and Csaki1973) values by forwards and backwards stepwise selection of all variables allowing adjusted OR and P-values to be calculated. To generate an experimental metric by village for comparisons of the utilization of health services, the affirmative responses for the questions 3, 4, 5, 6 and 7 were pooled and expressed as a percentage; these were plotted against the prevalence of STH at each village to ascertain covariance.
Relationships between worm length versus mass and average worm weight versus worm burden were investigated by bivariate scatter plots. Spearman-rank correlation coefficients were calculated and tested for statistical significance using STATA v10 (StataCorp LP, Texas USA).
Ethical permission
Approval for this study was granted by the medical research ethics committees of the Ministry of Health and Social Welfare, Zanzibar and London School of Hygiene and Tropical Medicine. Written informed consent was taken from each mother, and on behalf of their child, as either a signature or thumbprint in indelible ink.
RESULTS
Parasitological survey
A total of 322 mothers and 359 children, aged between 6 months and 5 years, were registered from the 10 villages shown in Table 1. The mean age of mothers was 30·9 and children 2·8 years. A slightly higher proportion of boys (54·6%) were registered within the mother and child pairs. Owing to different levels of community participation, enrolment of mother and child pairs varied between villages with a maximum of 98 in Mahonda and a minimum of 28 in Kizimbani (Fig. 1). Overall failure rate to supply stool was 16·3%, ranging from 6·7% in Mtende to 32·5% in Fujoni, and was equivalent between mothers and children.
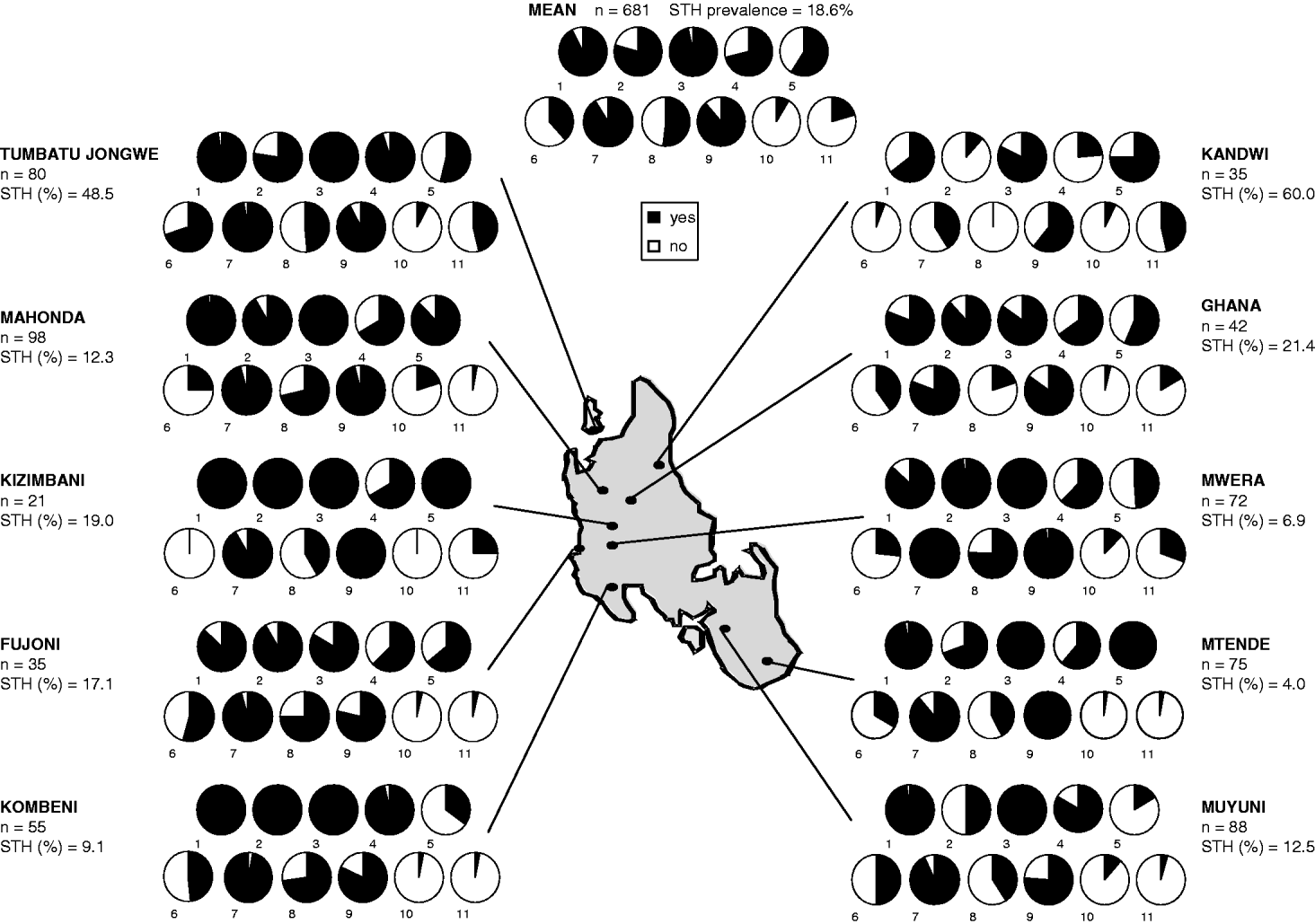
Fig. 1. Schematic map of Unguja Island depicting questionnaire responses at each of the 10 sampled villages together with overall mean reveals elevated prevalence of STH infection at Kandwi and Tumbatu Jongwe. At each village the numbers of mothers and children surveyed (n) and percentage of individuals with at least 1 STH infection are indicated. The 11 yes/no questions were as follows. (1) Do you know what STH is? (2) Does your household have a latrine? (3) Have you recently accessed local health services? (4) Have you ever taken STH treatment? (5) Has your child ever taken STH treatment? (6) Has your child had a Vitamin A supplement? (7) Has your child had immunizations? (8) Does your child usually wear shoes/sandals? (9) Does your child often play on the ground? (10) Has your child ever passed blood in stool? (11) Has your child ever passed worms in stool? Unguja is approximately 85 km long and between 20 and 30 km wide.
Table 1. Age and sex of study population with prevalence and intensity of STH
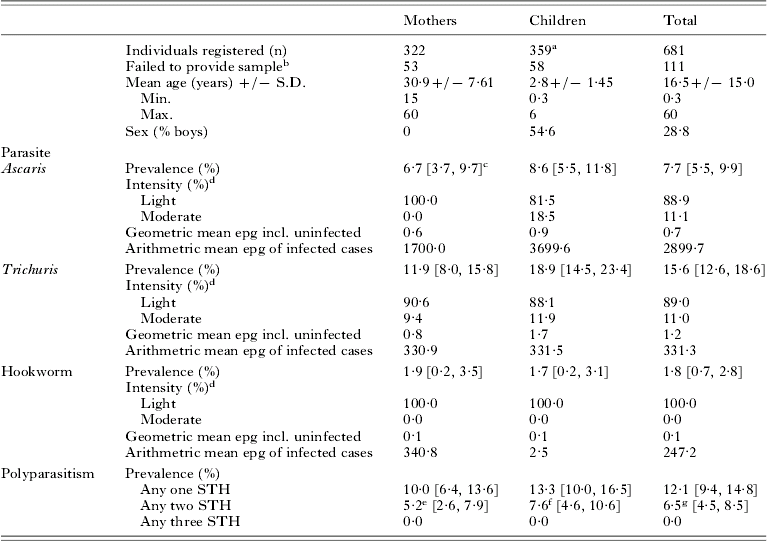
a Some mothers reported with further child siblings.
b Number of individuals who did not provide a stool sample for analysis.
c Values in parentheses are 95% confidence intervals.
d Intensity of infection classified according to WHO faecal egg count guidelines.
e 4·8% Ascaris and Trichuris; 0·4% Trichuris and hookworm co-infection.
f 6·3% Ascaris and Trichuris; 1·3% Trichuris and hookworm co-infection.
g 5·6% Ascaris and Trichuris; 0·9% Trichuris and hookworm co-infection.
General prevalence of Ascaris, Trichuris and hookworm was 7·7%, 15·6% and 1·8%, respectively. By village STH prevalence varied widely but can be largely explained by high infection rates in just 2 northern locations: Tumbatu Jongowe [Ascaris (39·4%) and Trichuris (40·9%)] and Kandwi [Ascaris (28·6%) and Trichuris (57·1%)] which fall outside the upper 95% confidence interval of the aggregated prevalence data. Infection intensities across the survey were predominantly light, only 11% of cases harboured moderate infections and none was heavy. Despite similar Ascaris infection rates in mothers (6·7%) and children (8·6%) the arithmetic mean Ascaris egg counts of infected children was over double that of infected mothers. The arithmetic mean EPG of infected children was skewed by 3 subjects from Kandwi that had moderate intensity infections. Geometric mean egg counts were less sensitive to these ‘outliers’ but relative values for mothers and children showed a higher infection intensity in children (Table 1). Using EPG data, aggregation of Ascaris infections within mothers (k=0·0073) was only slightly different from that within children (k=0·0079) giving a mean k of 0·0096.
The general prevalence of Trichuris in children (18·9%) fell outside the 95% upper confidence limit of prevalence in mothers (11·9%) and the geometric mean Trichuris egg counts of children is over twice that of mothers. Using EPG data, aggregation of Trichuris infections within mothers (k=0·0136) was increased to that of children (k=0·0263) and, when data were combined, an overall mean k of 0·0201 was obtained. Aggregation in hookworm was very similar in mothers (k=0·0024) and in children (k=0·0025), with an overall k of 0·0024, with overall k of 0·0021. General levels of STH aggregation could be ranked in the following ascending order: Trichuris, Ascaris and hookworm.
Mothers and children were at similar risk of co-infection, both being about half as likely to be co-infected with any two STH parasites as being infected with a single STH species. Concurrent Ascaris and Trichuris infections were relatively common amongst those infected (5·6%), accounting for 30·2% of all STH infections (Table 1). Multiple infections with hookworm and Trichuris were rare (0·9%) and no individuals were found to have concurrent hookworm and Ascaris infection.
Questionnaire data and risk factors
Fig. 1 illustrates general responses to questionnaires by village. Knowledge of STHs and access to health services is generally high across all villages, while history of STH treatment is varied. Reported treatment coverage was significantly higher in mothers than in children (OR=1·72, CI95 1·23–2·39, P<0·01) but with no correlation, at the individual level, between reported STH treatment and STH prevalence (P=0·23). About half of all children wore shoes/sandals but it is notable that none of the children in Kandwi possessed shoes, a village that had the highest prevalence of STH (60%). Kandwi also had the lowest number of mothers with reported access to latrines, less than 15%, whilst over 75% of mothers at 7 other villages reported to have access. Overall, prevalence of mothers reporting blood and worms in their child's stool was low, but reported worm expulsion correlated significantly with child's STH prevalence (OR=2·30, CI95 1·26–4·22, P<0·01).
Three variables: access to a latrine, having an infected family member and possessing shoes were found to be significantly associated with any STH infection. The presence of a latrine (OR=0·47, P<0·01) and possessing shoes (OR=0·46, P<0·019) were strongly negatively associated with STH infections. A greater negative association of latrines and STH was more strongly revealed in children than mothers. Having an infected family member was associated with a substantially increased risk of STH infection (OR=3·97, P<0·001) demonstrative of substantive associations of self-contaminating contact between mother and child. After an exhaustive investigation of multilevel logistic regression models, it was found that access to a household latrine (OR=0·56, 95% CI [0·32, 0·99] P=0·048) and having an infected family member (OR=3·72, 95% CI [2·22, 6·26] P<0·001) were most important predictors while adjusting for village effects.
Having access to health services (OR=0·57, P=0·505) and previous history of treatment (OR=1·36, P=0·217) did not significantly affect the odds ratio of STH infection. Having knowledge of STHs was not significantly associated with infection, with a decreased odds ratio (OR=0·79, P=0·566), however, this association almost became significant in mothers at the 5% level when controlling for other risk factors. The responses to 5 questions relating to accessing health services and receiving some form of treatment were pooled for each individual and aggregated by village. This gave an arbitrary health utilization metric for comparisons with STH prevalence by village (Fig. 2). The health utilization scores for 8 out of the 10 villages fell within 60% and 80% and associated prevalence of STH ranged from 4·0% to 21·4%; two villages were outliers to this group and their conflicting patterns of either high or low access to health services while having elevated STH prevalence is peculiar against the 7 other villages. Utilization of health services at Tumbatu Jongowe village was some 10% higher than average while at Kandwi village was some 10% lower than average yet prevalence of STH was 48·5% and 60·0%, respectively. There appeared to be a weak association (y=−0·002x+0·75) of decreasing health services utilization with an increasing prevalence of STH although this was not statistically significant (P>0·05). Finally, health scores were not correlated with infection prevalence in children (P=0·19), but were with infection prevalence in mothers, where mothers with increasing health scores were more likely to be infected with STHs (OR=1·69, CI95 1·11–2·59, P=0·015). Additionally, a correlation between health scores and locality was observed at Tumbatu Jongwe where individuals were more likely (OR=15·14, CI95 3·56–64·43, P<0·001) to have a higher health score (>3) than individuals from all other villages.
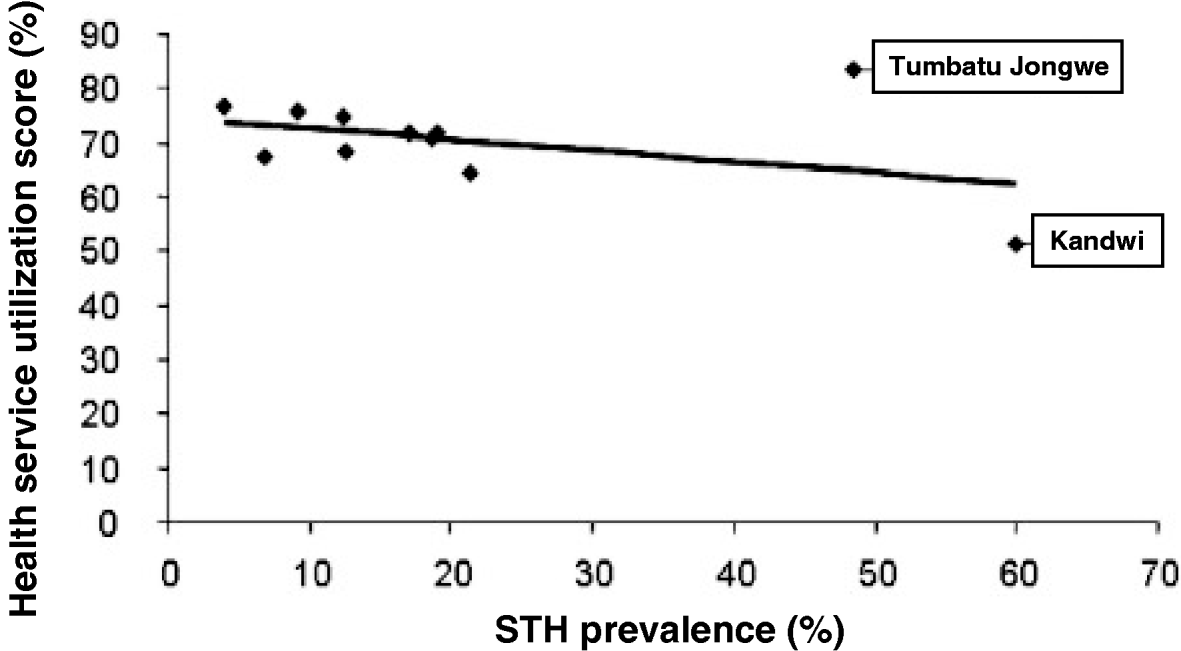
Fig. 2. Bivariate scattergram of the relationship between accessing health services scores and any STH infection; there appears to be a negative association between increasing prevalence of STH and decreasing utilization to health services. The health services utilization score is calculated as a pooled percentage of positive responses from questions 3, 4, 5, 6 and 7 in the questionnaire.
Analysis of Ascaris expulsion
Adult worms were collected from 16 children, the mean adult worm burden was 5·3 with a maximum of 17. Of the 68 worms sexed, female worms outnumbered male worms by nearly 2·5 times. Mean worm length was 17·1 cm with minimum of 6 cm and maximum of 31·0 cm, mean worm mass was 2·12 g with a minimum of 0·08 g and maximum of 7·14 g. Plotting worm length against mass revealed a positive and highly correlated power relationship (y=0·001x2·58) between worm length and mass (rp=0·936, n=68, P<0·001) (Fig. 3A). The relationship between the mean mass (g) of individual worms and the number of worm harboured in each host is shown in Fig. 3B. Mean worm mass was calculated by the number of worms collected from study subjects plotted against the total number of worms expelled. For worm burdens of differing intensity, there is strong evidence for a negative relationship between an increasing total number of worms yet decreasing mean worm mass.
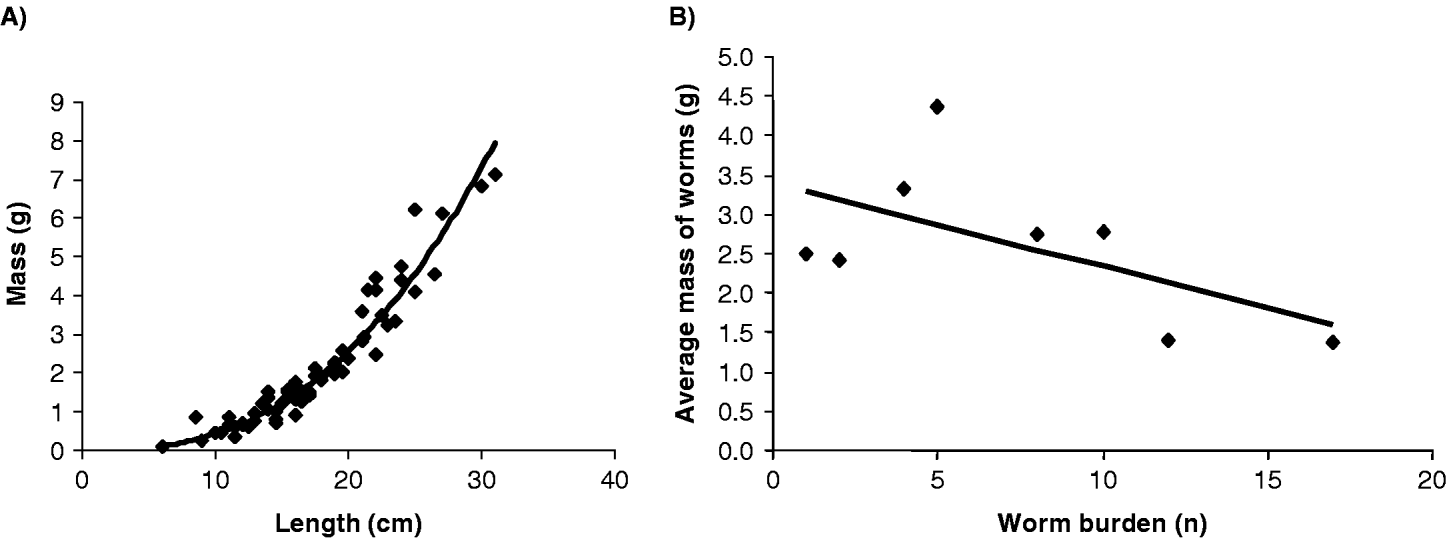
Fig. 3. Biometric investigations of collected Ascaris worms. (A) A clear non-linear relationship is apparent between worm mass (g) and worm length (cm) for the 68 worms measured (r=0·936, P<0·001). (B) A strong negative association was observed between mean worm mass for each infected person and individual worm burden (r=−0·381, P=0·014). This would imply that a density-dependent process is operating, for example, those worms originating from individuals with increasing worm burdens are proportionately lighter than those worms obtained from individuals harbouring fewer worms.
DISCUSSION
Gathering contemporary epidemiological data on STH in infants and pre-school children is essential for evaluation of ongoing interventions and more importantly for evidence-based direction of future control efforts (Goodman et al. Reference Goodman, Haji, Bickle, Stoltzfus, Tielsch, Ramsan, Savioli and Albonico2007; Albonico et al. Reference Albonico, Allen, Chitsulo, Engels, Gabrielli and Savioli2008). In such surveys of younger children it is essential to include mothers in parasitological surveillance for at least two reasons. Firstly, mothers are essential in obtaining informed guardian consent, assisting in the collection of the child's stool sample and answering questionnaires on behalf of their child. Secondly, it permits a better parasitological assessment of putative cross-contaminating behaviours between each mother and child pair as well as their local associated risk factors, for example, having access to a household latrine. Moreover, it also opens up a direct dialogue with each mother permitting an important health education access point for each household not only for STH but also for other communicable diseases.
This survey of some 322 mother and child pairs has shed new light upon the local importance of spatial heterogeneity of STH on Unguja. While general prevalence of any STH was 18·6% there were notable exceptions where prevalences were in excess of this, for example, Kandwi (60%), Tumbatu Jongowe (48·5%) and Ghana (21·4%); specifically the highest encountered STH prevalence was trichuriasis (57·1%) at Kandwi. These northern villages represent settlements within predominantly rural environments and although they appeared to have broadly similar accessibility to health services (see Fig. 2), there must be locally elevated infection risk factors; for example, no child in Kandwi village was reported to wear shoes/sandals while almost half of all surveyed children wore them. Shoes are well-known to offer protection against hookworm larvae (Crompton and Savioli, Reference Crompton and Savioli2007) although in this instance their absence and strong association with Ascaris and Trichuris infections is a reflection of local poverty. As another likely predictor of lower economic capacity, it is therefore not surprising that Kandwi village had the lowest number of mothers having access to household latrines (15%). Access to health services and previous history of treatment did not significantly affect the odds of STH infection, so it could be broadly assumed that the study population had a reasonable provision of regular medical care as mediated through MCH clinics (see Figs 1 and 2). Thus it is necessary to inspect other causal factors for higher STH prevalences at Timbatu Jongwe and Kandwi villages which fall well outside the upper 95% confidence interval of the aggregated prevalence data.
In addition to obvious sanitation factors, elevated STH prevalences in northern villages could be influenced by local environmental factors and some additional notes are pertinent here. The soils in these northern environments are of predominantly alluvial clays whereas in central and southern regions they are of sandy, coralline deposits (Stothard et al. Reference Stothard, Loxton, Rollinson, Mgeni, Khamis, Ameri, Ramsan and Savioli2000, Reference Stothard, Mgeni, Khamis, Seto, Ramsan, Hubbard, Kristensen and Rollinson2002a). Longevity of Ascaris and Trichuris eggs within alluvial clay soils contaminated by faecal material might be elongated in comparison to more sandy, coralline soils. Secondly, it is commonly known that on Unguja, like in nearby Kenya and Pemba (Luoba et al. Reference Luoba, Geissler, Estambale, Ouma, Magnussen, Alusala, Ayah, Mwaniki and Friis2004, Reference Luoba, Geissler, Estambale, Ouma, Alusala, Ayah, Mwaniki, Magnussen and Friis2005; Young et al. Reference Young, Dave, Farag, Said, Khatib, Sabra, Tielsch and Stoltzfus2007) geophagia or pica (eating clays/soils) takes place; a recent survey has found that 20% of adults in the north practice pica while only 1% do the same in the south (J. R. Stothard, unpublished observations). As pica can be especially common amongst pregnant women (Luoba et al. Reference Luoba, Geissler, Estambale, Ouma, Alusala, Ayah, Mwaniki, Magnussen and Friis2005) this behavioural component, practiced within a permissive environment, could enhance local transmission increasing the intensity of faecal egg ingestion and shortening the re-infection period. In future, it would be strongly advisable to gather further information on patterns of local geophagy and environmental contamination not only to better estimate individual risk but also to devise local strategies for initiating behavioural change for amelioration.
The levels of parasite aggregations within the mother and child population showed interesting trends between helminth species. Across the sampled mother and child population, an increasing aggregation of each STH species was detected: Trichuris (k=0·0201), Ascaris (k=0·0076) and hookworm (k=0·0024). In comparison with other epidemiological studies these values are generally 2 orders of magnitude smaller than that reported by Martin et al. (Reference Martin, Keymer, Isherwood and Wainwright1983). This is likely caused by previous rounds of chemotherapy in the region clearing infection in the lightly-infected individuals and the remaining worm burden becoming concentrated in a smaller subset of the population. While levels of aggregation for Ascaris and hookworm were roughly equivalent in mothers and children those for Trichuris, however, showed lesser aggregation in mothers than children. Taking available data from the 2006 survey of Kick out Kichocho where approximately 4000 children from 24 schools have been surveyed, present values of k for each STH species were as follows: Ascaris (k=0·0079), Trichuris (k=0·0134) and hookworm (k=0·0030) (French, 2006). Overall these values of k were in fair alignment with the mother and child data.
The intensities of infection, as quantified by EPG and classified according to WHO (Montresor et al. Reference Montresor, Crompton, Bundy, Hall and Savioli1998) were predominantly ‘light’ across Unguja for both mothers and children. The relatively few cases of moderate Ascaris and Trichuris infections correlated with villages of elevated prevalences and this could be seen as good evidence of the successful impact of MDA depressing general infection intensities. Still light infections in naïve children are thought to suppress protein metabolism, appetite and erythropoiesis by triggering an inflammatory cytokine response (Stoltzfus et al. Reference Stoltzfus, Chway, Montresor, Tielsch, Jape, Albonico and Savioli2004), therefore continuing control initiatives is important to keep STH at these low levels and provide benefit to the health of the child (Albonico et al. Reference Albonico, Allen, Chitsulo, Engels, Gabrielli and Savioli2008).
It should not be forgotten, however, that prevalences and intensities reported here are likely to be underestimates, downwardly biased by the insensitivity of single Kato-Katz examinations (Goodman et al. Reference Goodman, Haji, Bickle, Stoltzfus, Tielsch, Ramsan, Savioli and Albonico2007). In terms of actual Ascaris adult worm burdens it appears that infections encountered so far were usually less than 15 worms per individual and while these are similar to numbers reported by others (see Chan et al. Reference Chan, Bundy and Kan1994a, b) it is much lower than that reported by Hall and Holland (see Hall et al. Reference Hall, Anwar, Tomkins and Rahman1999; Hall and Holland, Reference Hall and Holland2000). The mass of individual worms was negatively correlated (rs=−0·381, n=8, P=0·014) against worm burdens and is suggestive of a density-dependent mechanism similar to that reported by Elkins and Haswell-Elkins (Reference Elkins and Haswell-Elkins1989). Typically, adult Ascaris worms measure between 15 and 40 cm (Crompton, Reference Crompton2001; Crompton and Savioli, Reference Crompton and Savioli2007) and it is of note that 23 (33·8%) of the worms measured in this study fell below this range. This might indicate that a relatively high proportion of worms were immature, yet most were gravid, maybe this could be interpreted as the beginnings of an adaptive survival strategy of Ascaris in response to regular chemotherapy. For example, repeated rounds of regular treatment might progressively select for populations of worms which could more quickly reach fertility for a given size. This subtle change in life history of maturation times could happen even in the face of undiminished anthelminthic performance.
To conclude, this study has provided contemporary information on the present situation of STH in infants and pre-schoolchildren and their mothers and drawn attention to the importance of local sanitation and human behavioural factors as fundamental forces influencing the success of regular MDA at the local level. Villages in the north of Unguja appear to represent locations where there is greater transmission potential of STH and, within these settings, a more inclusive control strategy, bringing in health and hygiene education for example, is needed for MDA to have a more enduring impact.
We thank the shehias, mothers and children who took part in this survey. We are grateful to the Zanzibar MoHSW for their continuing assistance for the Kick out Kichocho programme. In addition we would like to thank Ms Laura Ambrose, LSHTM, Ms Elissa Klinger, Harvard University and Ms Fiona Allan, NHM, for their assistance in the field as well as to the technical support staff of the HCLU for assistance in the laboratory. We gratefully acknowledge programme support from the Schistosomiasis Control Initiative, UK and the African Development Bank, Tanzania. This work was financially supported by The Health Foundation, UK.






