Introduction
Ticks (Parasitiformes: Ixodida) are distinctive arachnids, all of which are haematophagous ectoparasites of vertebrates. As important vectors of several diseases in humans and livestock, they have attracted a considerable body of research (Sonenshine and Roe, Reference Sonenshine and Roe2013). Approximately 905 living species are conventionally divided into ~714 hard ticks (Ixodidae), ~190 soft ticks (Argasidae), plus a further family (Nuttalliellidae) with a single species (Beati and Klompen, Reference Beati and Klompen2019). Fossil ticks are rare but have occasionally been recorded as subfossils assignable to living species (Sanchez et al., Reference Sanchez, Nava, Lareschi, Ortiz and Guglielmone2010). Most tick fossils are inclusions in amber. Both hard and soft ticks are known from Miocene Dominican Republic amber (Lane and Poinar, Reference Lane and Poinar1986; Poinar, Reference Poinar1995), dated 20–15 Ma (Peris et al., Reference Peris, Solórzano Kraemer, Peñalver and Delclòs2015). There is a hard tick from Eocene (ca. 49–44 Ma) Baltic amber and a soft tick from Late Cretaceous (ca. 94–90 Ma) New Jersey amber (Weidner, Reference Weidner1964; Klompen and Grimaldi, Reference Klompen and Grimaldi2001). The oldest, and most productive, source of fossil ticks is the mid-Cretaceous (ca. 100 Ma) Burmese amber of Myanmar. This deposit hosts a surprisingly diverse fauna including two extinct genera of hard ticks, Cornupalpatum (Poinar and Brown, Reference Poinar and Brown2003) and Compluriscutula (Poinar and Buckley, Reference Poinar and Buckley2008), alongside fossils assigned to two living hard tick genera, Amblyomma Koch, 1844 and Haemaphysalis Koch, 1844 (Klompen in Grimaldi et al., Reference Grimaldi, Engel and Nascimbene2002; Chitimia-Dobler et al., Reference Chitimia-Dobler, Cancian de Araujo, Ruthensteiner, Pfeffer and Dunlop2017, Reference Chitimia-Dobler, Pfeffer and Dunlop2018). There is also an extinct family and genus: Deinocrotonidae and Deinocroton Peñalver, Arillo, Anderson and Pérez-de la Fuente, 2017 (Peñalver et al., Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017).
Hard ticks are further subdivided into two clades: Prostriata, containing the genus Ixodes Latreille, 1795 (Latreille, Reference Latreille1795), and Metastriata encompassing the remaining Ixodidae genera. Prostriates and metastriates can be distinguished on characters such as the position of the groove around the anus and the absence or presence of festoons around the posterior edge of the body. All hard ticks found in Burmese amber so far have been metastriates, while the oldest prostriate is an Ixodes species from Baltic amber (Weidner, Reference Weidner1964; Dunlop et al., Reference Dunlop, Apanaskevich, Lehmann, Hoffmann, Fusseis, Ehlke, Zachow and Xiao2016). Here, we describe the first Ixodes tick from Burmese amber doubling the stratigraphic range of Prostriata. The second inclusion represents the first adult female of Cornupalpatum in Burmese amber and, like a previous record (Peñalver et al., Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017), is associated with a dinosaur feather which has implications for its feeding ecology. A third inclusion represents a new species belonging to the previously described extinct genus Deinocroton. The final and most surprising inclusion is even more interesting having a body resembling that of a soft tick, but a capitulum (the region bearing the mouthparts) like that of a hard tick. This latter inclusion represents an extinct lineage, potentially ancestral to the two main tick families today. However, molecular data suggest that the split between Ixodidae and Argasidae was considerably older than the mid-Cretaceous (Mans et al., Reference Mans, de Klerk, Pienaar, de Castro and Latif2012, Reference Mans, Featherston, Kvas, Pillay, de Klerk, Pienaar, de Castro, Schwan, Lopez, Teel, Pérez de León, Sonenshine, Egekwu, Bakkes, Heyne, Kanduma, Nyangiwe, Bouattour and Latif2019), which could imply that the new fossil is a late survivor of an earlier radiation.
Materials and methods
Material
Three fossils originate from the private collection of Patrick Müller and bear the specimen numbers BUB4185 (Ixodes antiquorum sp. nov.), BUB4029 (Khimaira fossus sp. nov.), BUB3319 (Deinocroton copia sp. nov.). One is from the collection of Lidia Chitimia-Dobler (the Cornupalpatum female). Specimens from Patrick Müller have been deposited in the Paleontological collection in Munich (BUB4185: SNSB-BSPG 2021 XII 10; BUB4029: SNSB-BSPG 2021 XII 11; BUB3319: SNSB-BSPG 2021 XII 12) and from Lidia Chitimia-Dobler in the Museum für Naturkunde Berlin.
Imaging
For photography, a Keyence VHX-7000 Digital Microscope with an FI 4K Revolver Head (Keyence Itasca, IL, USA), an X-Y and Z-motorized stage, and a tiltable stand, with a combination of incident and transmitted light for focus stacking and a Keyence VHX-900F (Keyence Itasca), were used. Magnifications ranged from 100 to 1000 times. Polarized light was used for some images to resolve more details, and the resulting image stacks were combined using the software Helicon Focus 6.7.1. Microscopic computed tomography (microCT) scans were acquired using a Zeiss XRadia MicroXCT-400 (Carl Zeiss X-ray Microscopy, Pleasanton, CA, USA). Acquisition settings were adapted depending on the size of the specimen, size of the amber piece and required level of detail. For the D. copia sp. nov. specimen, the whole body was scanned at 80 kVp per 100 μA with 30 s exposure using the 0.4× detector assembly resulting in 4.64 μm isotropic voxel size. For the K. fossus sp. nov. specimen, the whole body was scanned at 80 kVp per 100 μA with 30 s exposure using the 4× detector assembly resulting in 1.95 μm isotropic voxel size. For the I. antiquorum sp. nov. specimen, capitulum and scutum were scanned at 80 kVp per 50 μA with 60 s exposure using the 20× detector assembly resulting in 0.44 μm isotropic voxel size. For the Cornupalpatum female, the whole body was scanned at 40 kVp per 200 μA with 30 s exposure using the 4× detector assembly resulting in 1.37 μm isotropic voxel size. All scans were recorded over a 360° specimen rotation with an angular increment of 0.225° between projections. Image volumes were processed and visualized by volume rendering using the 3D software package Amira 6.4. Drawings were prepared with a camera lucida attachment on a Leica M205C stereomicroscope (Leica Microsystems, Wetzlar, Germany), again using a combination of incident and transmitted light where appropriate.
Results
A new prostriate fossil
Class Arachnida Lamarck, Reference Lamarck1801
Order Parasitiformes Reuter, Reference Reuter1909
Suborder Ixodida Leach, Reference Leach1815
Family Ixodidae Murray, Reference Murray1877
Ixodes Latreille, Reference Latreille1795
Ixodes antiquorum Chitimia-Dobler, Mans and Dunlop sp. nov.
Etymology. From the Latin antiquus (aged, ancient).
Holotype. Female tick (BUB4185) (Fig. 1) deposited in the Munich Paleontological Collection. The species name was registered with Zoobank (LSID code: zoobank.org:act:739BD930-5C1C-4E6E-8C4A-A05180B216A8).
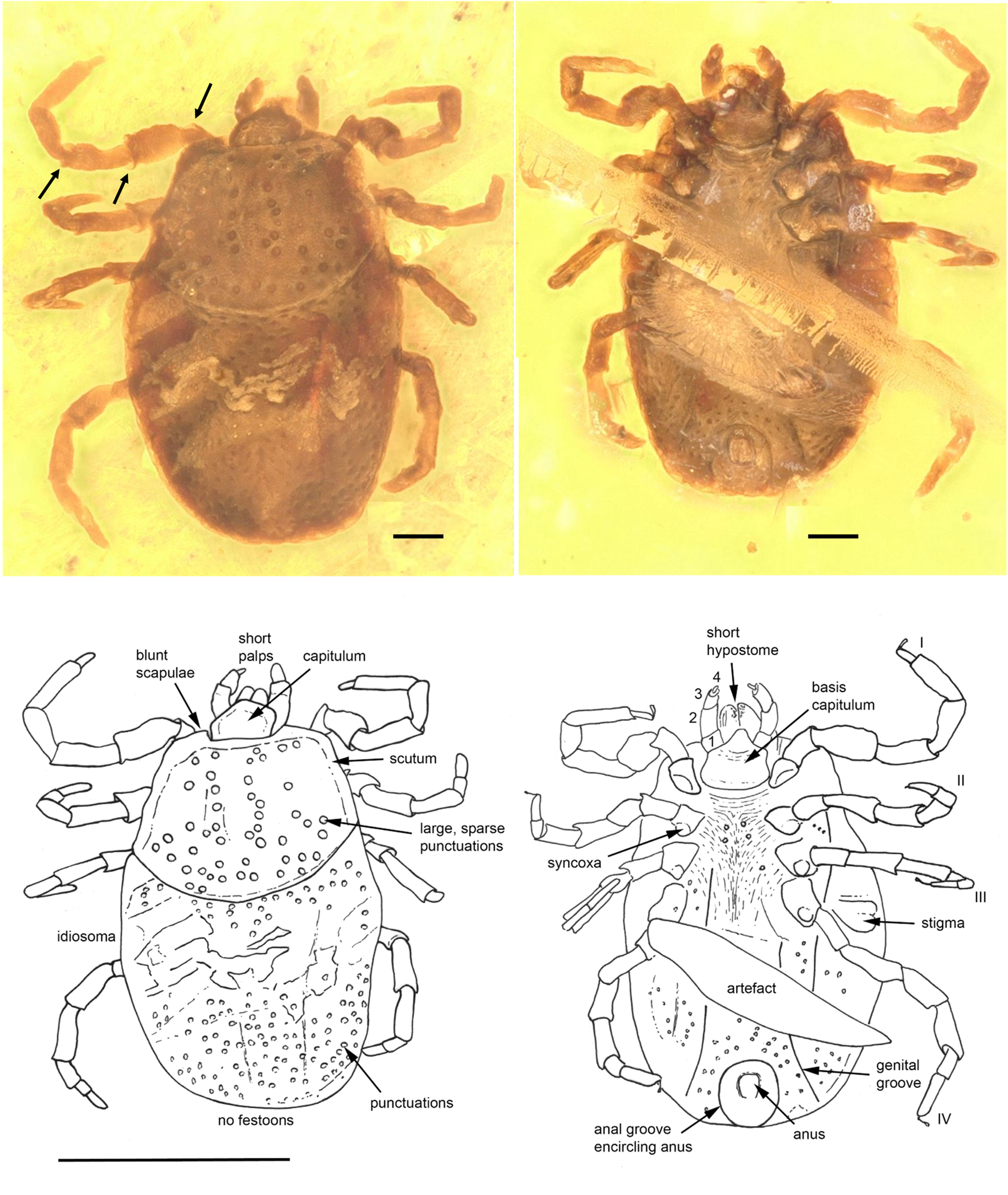
Fig. 1. Ixodes antiquorum sp. nov. (Ixodidae) from Burmese amber designated as the holotype for this species. Indicated are dorsal (left) and ventral (right) images. The absence of festoons and the anterior anal groove can be clearly discerned. Arrows indicate the presence of notch-like processes on the joints. Line drawings at the bottom indicate important aspects described in the text. Scale bars indicated are 0.1 mm for the photos and 1 mm for line drawings.
Diagnosis. Ixodes nymphal tick in which the anal groove encircles the anus anteriorly, eyes absent, festoons absent, coxae without spurs. Scutum wider than long, carinae absent on basis capituli and scutum; trochanter, femur and genu articulations with notch-like processes.
Description of nymph
Idiosoma: body ovoid, length from middle of scutum to posterior body margin: 0.793 mm; maximum width (measured in middle, behind third legs) 0.565 mm; dorsal and ventral surface without setae, but with a moderate number of large punctuations (Fig. 1). Scutum 0.471 mm wide (measured in middle) and 0.342 mm long (from middle to edge); broadest prior to the posterior end, large sparse punctuations distributed throughout scutum, sides straight and diverging posteriorly, posterior margin slightly convex, posterior corrugations absent; scapulae blunt (Fig. 1). Cervical grooves not visible (Fig. 1). Anus visible, median; anal groove encircling anus anteriorly and converging posteriorly (Fig. 1). Genital groove visible only at posteroventral edge of idiosoma. Stigma subtriangular with small macula (transverse axis 0.120 mm by 0.059 mm) behind right IV coxa.
Capitulum: Length from apices to the posterior margin of basis 0.134 mm. Basis capituli almost rectangular dorsally with posterolateral margins a little divergent anteriorly; posterior margin straight, cornuae absent; ventrally rectangular, posterior margin rounded, length from palpal insertion to the posterior margin of basis 0.064 mm, width 0.151 mm, no auriculae. Palpi short, thick, convex dorsally, much separated at the base, with long axes converging in front; four articles with lengths: trochanter 1, 0.023 mm; femur 2, 0.059 mm; genu 3, 0.040 mm; tibiotarsus 4, 0.017 mm. Hypostome short, bluntly rounded apically, 0.096 mm in length, denticles arranged in 3–4 rows from top to bottom. More detail on the file and number of denticles was not available due to the presence of host-derived tissue on the hypostome. Chelicerae well developed, equal in length to hypostome.
Legs: Coxae subtriangular, internal and external spurs absent, syncoxae present on all coxae. Tarsus I gradually stepped and tarsi II–IV stepped. Trochanter, femur and genu joints of all legs have notch-like processes, and spurs dorsally and ventrally (Fig. 1).
Chaetotaxy: Two setae observed on all leg articles, and four setae associated with Haller's organ. Palps bear small setae on the femur and 4–5 small setae on genu proximate to the joint with the tibiotarsus.
Remarks. Ixodes are currently subdivided into 16 subgenera (Clifford et al., Reference Clifford, Sonenshine, Keirans and Kohls1973; Robbins and Keirans, Reference Robbins and Keirans1992; Durden and Keirans, Reference Durden and Keirans1996). Our new fossil species cannot be placed with confidence in any particular living subgenus as it possesses morphological features consistent with a number of different taxa. The fossil shares a number of morphological aspects with the members of the subgenus Endopalpiger and Exopalpiger: broader than longer scutum with sparse large punctuations (not dense as in Ixodes tasmani Neumann, 1899), blunt scapulae, scutum carinae and cornua absent, and the anal groove and coxae are quite similar. Morphological characters shared with nymphs of Ixodes holocyclus Neumann, 1899 (Sternalixodes) include the trochanter small, round and somewhat salient laterally but visible only ventrally. The sternal plate is absent in the nymph but can be present in females (Durden and Keirans, Reference Durden and Keirans1996). The presence of syncoxae in the fossil is a morphological character observed in adults of some species from the subgenus Endopalpiger and in adults and nymphs of some Sternalixodes species (Roberts, Reference Roberts1960). These ticks possess a type of scutum, broad posteriorly, which appears to be somewhat characteristic of Australian forms. It is observable in Ixodes australiensis Neumann, 1908, Ixodes ornithorhynchi Lucas, 1845 and I. tasmani, and the scutum of the nymph of Ixodes vestitus Neumann, 1908 is of this shape (Roberts, Reference Roberts1960). Like the fossil, some Australian living Ixodes species cannot be easily placed in a subgenus, such as Ixodes barkeri Barker, Reference Barker2019; Ixodes heathi Kwak, Madden and Wicker, 2018; Ixodes woylie Ash et al., 2017; and Ixodes laridis Heath and Palma, 2017 (Barker, Reference Barker2019) based on morphological features.
The hypostome of the fossil could not be described in detail due to a piece of soft tissue from the host that is still attached to this structure (Supplementary Fig. 1). This is the first observation of soft tissue still attached to the hypostome of a fossil tick. The presence of an artefact identified as a possible mammalian hair (Fig. 1) is also of interest and is suggestive of a possible host for this tick species.
The first female fossil for Cornupalpatum
Family Ixodidae Murray, Reference Murray1877
Cornupalpatum burmanicum Poinar and Brown, Reference Poinar and Brown2003
Description of unengorged adult female
Idiosoma: Ornamentation indistinct; body subcircular, length from middle of scutum to posterior body margin: 1.392 mm; maximum width (measured in middle, behind third legs) 1.435 mm; dorsal and ventral surface without setae, but with moderate number of small punctuations (Fig. 2). Scutum can be seen only on the posterior part and seems to be subtriangular (Fig. 2). Eleven festoons. Anus visible, median; anal groove behind the anus, well visible, large ‘V’ shape (Fig. 2). Genital aperture median, forming transverse slit with the edges twisted inward, like a loop, situated between coxae III; spiracle plates comma-shaped, medial and lateral margins parallel, dorsal prolongation long, broad, perpendicular to the anterior–posterior axis, macula, round, situated subterminally; genital groove absent.

Fig. 2. A fossil of a female Cornupalpatum burmanicum (Ixodidae). Indicated are dorsal (up) and ventral (down images). The genital aperture, anus and the posterior V anal groove can be clearly discerned. A dinosaurian feather can be seen on the dorsal side. A line drawing at the bottom indicates important features. The scale bar in all figures is 1 mm.
Capitulum: Length from apices to the posterior margin of basis 0.388 mm; basis capituli posterior margin straight ventrally, hooks on the internal sides of the genu; hypostome length 0.258 mm, columns of teeth on hypostome are 2/2 blunt-tipped teeth, with internal line 6 teeth and external line with 7 teeth; apical end like a wide blade with well-developed lateral hooks oriented anteroposteriorly (Supplementary Fig. 2).
Legs: Coxae I–IV with no obvious spurs; tarsus I tapering distally, clear, oval area on the tarsi I dorsum is Haller's organ; claws paired, slender, simple, slightly curved; with distinct pulvillus visible on some legs.
Chaetotaxy: small setae visible on some legs joint and tarsi I; long setae on the third palpal segment and around the Haller's organ were observed.
Remarks. The present specimen is the first adult female of this species and, like a previous record by Peñalver et al. (Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017), it is associated with a feather. On the dorsal side of the tick is the barb of a pennaceous feather. It is 5.903 mm long. Parts of the many barbules are broken; nevertheless, on the distal part of some barbules, hooklets can be seen. The barbules share similar morphology in their attaching base. Distal ramus and the barbules from one side are not visible, probably damaged before having become embedded in the resin. One of the claws on the first leg of the tick fossil grasps a barb from another feather (Supplementary Fig. 3). This provides further support for the hypothesis that C. burmanicum used feathered dinosaurs as hosts (Peñalver et al., Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017): both as an immature tick and now potentially as an adult female.
A new Deinocroton species
Family Deinocrotonidae Peñalver, Arillo, Anderson and Pérez-de la Fuente, 2017
Deinocroton Peñalver, Arillo, Anderson and Pérez-de la Fuente, 2017
Deinocroton copia Chitimia-Dobler, Mans and Dunlop sp. nov.
Etymology. From the Latin copia (abundance) to describe the apparent species abundance of this genus in the Myanmar amber deposits.
Holotype. Female (BUB3319) (Fig. 3) deposited in the Munich Paleontological Collection. The species name was registered with Zoobank (LSID code: zoobank.org:act:FEB6E4CC-BE4F-4764-9E23-98F41505DE43).
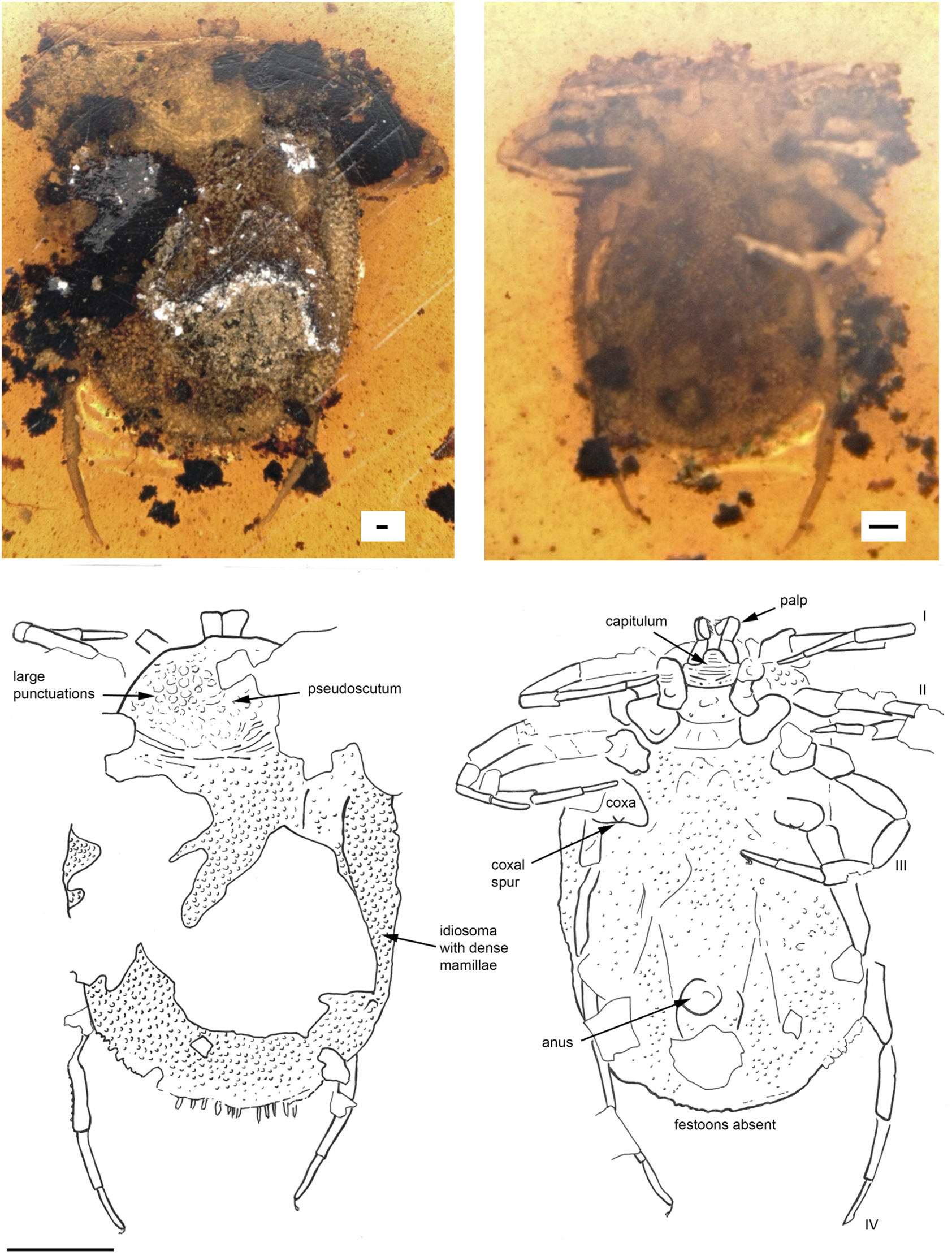
Fig. 3. Deinocroton copia sp. nov. (Deinocrotonidae) in Burmese amber designated as the holotype for this species. Indicated are dorsal and ventral views. Line drawings indicate pertinent features. The scale bars for the photos are 0.1 mm and for the line drawing 1 mm.
Diagnosis. Female D. copia sp. nov. possess on coxae I a single median spur, coxae II two spurs, medial posterior and distal anterior, and only a single small anterior spur on coxae III and a small median blind spur on coxae IV. Genital aperture between coxae II.
Description of female
Idiosoma: body subcircular; length from middle of pseudoscutum to posterior body margin: 4.018 mm; dorsal and ventral surface with dense mammillae, without discs or sutural line between dorsal and ventral surface (Fig. 3). Pseudoscutum in anterodorsal view is posteriorly broadened, 1.346 mm wide (measured in middle) and 1.063 mm long (from middle to edge); with large punctuations on the lateral sides, cervical grooves absent (Fig. 3); genital aperture between coxae II; anus and spiracles not visible. Eyes and festoons absent.
Capitulum: Capitulum not visible in dorsal view, rectangular ventral 0.180 mm wide and 0.441 long, boarded by the coxae I; hypostome subterminal not well visible; trochanter short and robust, femur longest, distally thickened in width and height, and with a blade-like formation in the middle of the internal side, genu bent ventral direction (creating a ventral concavity, with the surface of the femur) from the joint with the femur, wide and with spinous and transverse processes, tibiotarsus shorter than the femur, sword-like (Fig. 3, Supplementary Video).
Legs: Coxae well developed; coxae I and IV with a median spur, coxae II with two spurs; coxae III with small external spur; trochanter, femur, genu and tarsi articulations with notch-like processes. Dorsal and ventral edges of femur, genu, tibia and tarsi riffled (Supplementary Fig. 4).
Chaetotaxy: No visible setae.
Remarks: Characters that differentiate D. copia sp. nov. from D. draculi derive mainly from the number and locality of the spurs found on the coxae. For coxae I, both species possess a single median spur. For coxae II, D. copia possesses two spurs, medial posterior and distal anterior, while D. draculi possesses three spurs, two medial and one distal anterior. For coxae III and IV, D. draculi presents three spurs, two basal and posterior, and one medial anterior. Deinocroton copia sp. nov. only presents a single small anterior spur on coxae III and a small median blind spur on coxae IV. Genital aperture between coxae II in D. copia and between coxae II and coxae III in D. draculi.
A new tick family
Family Khimairidae Chitimia-Dobler, Mans and Dunlop fam. nov.
This family name was registered with Zoobank (LSID code: zoobank.org:act:E8C0A1D8-1364-46D9-A052-33E700E4FEE8).
Diagnosis. Nymphs with soft body, terminal gnathostoma, dense mammillae on body surface, discs on main body absent, scutum present, sutural line between dorsal and ventral surface and tarsal dorsal humps absent, pulvillus poorly developed.
Khimaira Chitimia-Dobler, Mans and Dunlop gen. nov.
The genus name was registered with Zoobank (LSID code: zoobank.org:act:EE3B8A7C-4470-4D25-9A4C-3ECF01DAB19C).
Etymology. From the ancient Greek khímaira (χῐ́μαιρᾰ), a mythological animal combining parts of more than one creature.
Diagnosis. As for the family.
Khimaira fossus Chitimia-Dobler, Mans and Dunlop gen. et sp. nov.
Etymology. From the Latin fossō (dig), as in a fossil.
Holotype. Nymph (BUB4029) (Fig. 4) deposited in the Munich Paleontological Collection. The species name was registered with Zoobank (LSID code: zoobank.org:act:BED4E24E-8477-4810-9259-F693037FB37D).
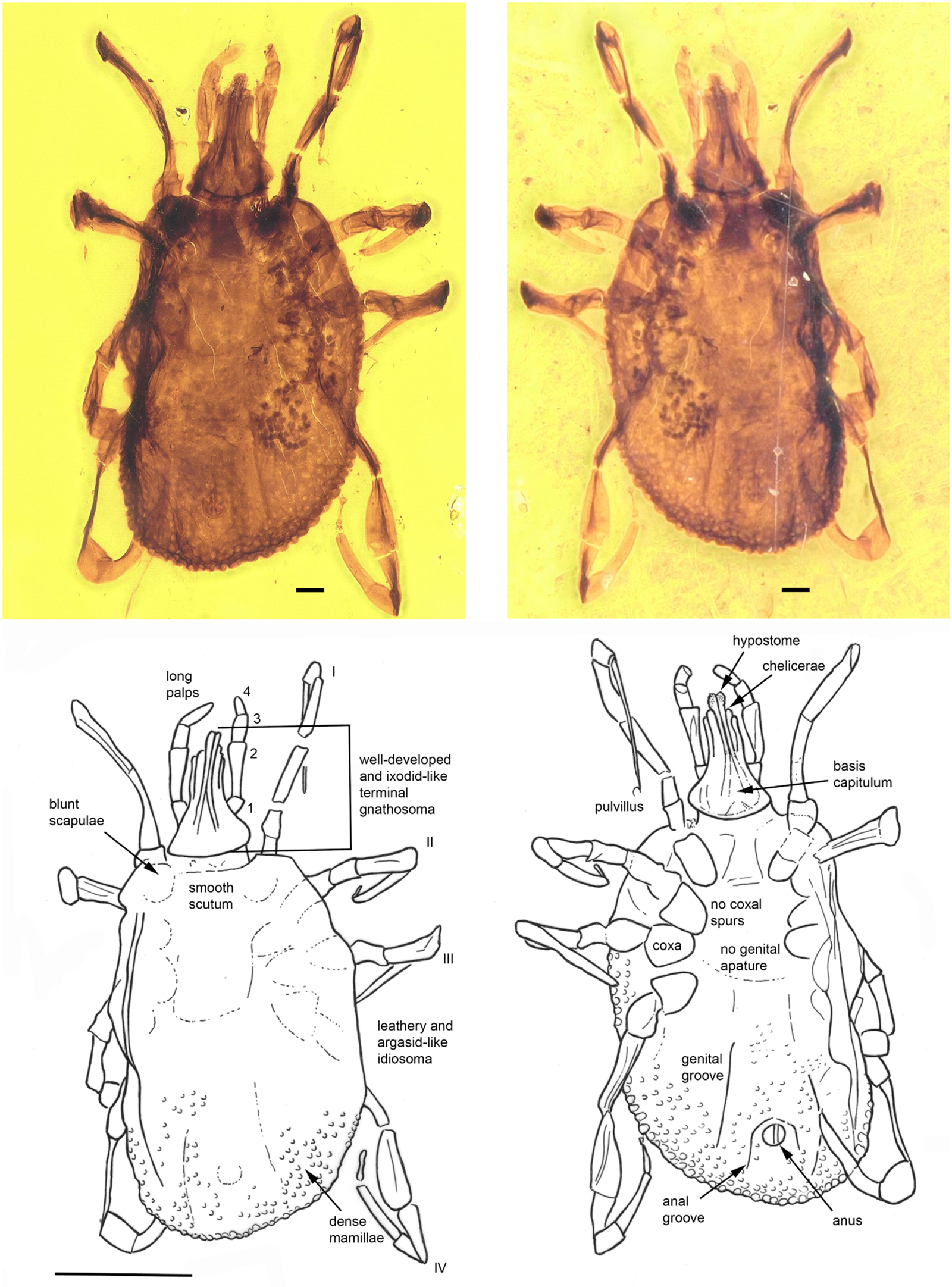
Fig. 4. Khimaira fossus gen. et sp. nov. (Khimairidae) in Burmese amber designated as the holotype for this species and the family Khimairida. The terminal gnathostoma, scutum and mammillated alloscutum can be clearly discerned. The line drawing indicates pertinent features. The scale bars for the photos are 0.1 mm and for the line drawing 1 mm.
Diagnosis. As for the family.
Description of nymph
Idiosoma: body oval, length from middle of scutum to posterior body margin: 1.277 mm; dorsal and ventral surface with dense mammillae, without discs or sutural line between dorsal and ventral surface (Fig. 4). Scutum subtriangular, 0.703 mm wide (measured in middle) and 0.522 mm long (from middle to edge); scapulae blunt (Fig. 4). Anus visible, median on the posterior part of idiosoma; anal groove slightly visible, encircling the anus anteriorly closing the sides above the idiosoma edge (Fig. 4). Stigmas located between coxae III and IV, broadly oval, longer axis transverse 0.155 mm × 0.095 mm. Genital aperture absent; genital groove visible posteriorly, on the side of anal groove. Eyes and festoons absent.
Capitulum: Length from apices to the posterior margin of basis 0.534 mm. Basis capituli outline roughly triangular, length from palpal insertion to the posterior margin of basis 0.127 mm, width 0.298 mm, no auriculae. Palpi long; four articles with lengths: trochanter, 0.074 mm; femur, 0.200 mm; genu, 0.093 mm; tibiotarsus, 0.115 mm. Hypostome arising from a flared anterior extension of the basal ‘collar’ of the capitulum, extending to below the level of chelicera distal end and the anterior third of the femur; apex bluntly pointed; dental formula 2/2; chelicera shorter than hypostome.
Legs: long, slender; coxae generally narrow, elongate oval, without spurs; tarsi gradually stepped, without humps; claws long, slender, simple, pulvilli poorly developed (Fig. 4).
Chaetotaxy: No visible setae.
Remarks. This fossil is interpreted as a nymph as it has four pairs of legs, but no genital aperture or porose areas which are specific characters for adult females. It is not a male as it presents a smooth scutum only on the anterior part of the idiosoma. The idiosoma has a leathery cuticle composed of innumerable small mammillae and lacks a lateral sutural line, thus resembling the cuticle of living Ornithodoros ticks in the family Argasidae. The intermammillary space and discs being absent further render it similar to nymphs of the Ornithodoros moubata group (Bakkes et al., Reference Bakkes, de Klerk, Latif and Mans2018). The stigmata that are located between coxae III and IV are also similar to soft ticks, compared to hard ticks where the stigmata are located behind coxae IV.
Despite the similarities to the Ornithodorinae, a remarkable feature of K. fossus gen. et sp. nov. is the fact the gnathosoma is in a terminal position; a character otherwise only seen in ixodid ticks. The gnathosoma of the new species is well-developed and has the second article of palps two times longer than articles 1 and 3. This is specifically seen in extant ixodids belonging to the genus Amblyomma (Nicholson et al., Reference Nicholson, Sonenshine, Lane, Uilenberg, Mullen and Durden2009). The fossil also has a scutum, a feature unique to hard ticks although it is difficult to determine whether the composition of the scutum is sclerotized as observed in hard ticks, or whether it is closer to the semi-sclerotized pseudo-scutum observed for N. namaqua and D. draculi (Latif et al., Reference Latif, Putterill, de Klerk, Pienaar and Mans2012; Peñalver et al., Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017). The capitulum of the new fossil has an extended collar around the chelicera and hypostome, similar to larvae of Carios quadridentatus (Heath, Reference Heath2012). The chelicerae are partly visible and seem to have an outer and inner digitus and are shorter than the hypostome of N. namaqua (Latif et al., Reference Latif, Putterill, de Klerk, Pienaar and Mans2012). The mammillated character of the integument differs from that of the Deinocrotonidae and Nuttalliellidae which both present a wrinkled integument with closely spaced pits. Taken together, an argasid-like body with ixodid-like mouthparts represents a unique combination of characters which merit a new, extinct family.
Discussion
Burmese amber originates from the Hukawng Valley in the Kachin State of northern Myanmar. It has been interpreted as a tropical forest environment (Grimaldi et al., Reference Grimaldi, Engel and Nascimbene2002) and is usually dated to the mid-Cretaceous, probably Upper Albian to Lower Cenomanian, or about 100 Ma (Shi et al., Reference Shi, Grimaldi, Harlow, Wang, Wang, Yang, Lei, Li and Li2012; Smith and Ross, Reference Smith and Ross2018). Burmese amber hosts a rich fauna (Ross, Reference Ross2019, Reference Ross2020), predominantly terrestrial arthropods. Debate remains about the precise palaeogeographic position of the locality during the time of amber deposition (Poinar, Reference Poinar2018), which impacts on the question to what extent the flora and fauna had their origins in Gondwana or Laurasia. Westerweel et al. (Reference Westerweel, Roperch, Licht, Dupont-Nivet, Win, Poblete, Ruffet, Swe, Thi and Aung2019) suggested that the Burma terrane was an island within the Trans Tethyian Arc during the mid-Cretaceous. In detail, the Burma terrane forms part of the Incertus Arc that formed ca.155 Ma and was linked to northern Australia and India via the Woyla Arc (Hall, Reference Hall2012). This connection could have provided a short window of land bridges for colonization by Gondwanan elements before the land bridges were destroyed at ca.140 Ma (Hall, Reference Hall2012). Continued northward movement would then place the Burma terrane in the Trans Tethyian Arc by the time of Burmese amber deposition at ca.100 Ma.
Burmese amber hosts the oldest known ticks, as well as the oldest records of two other members of the wider Parasitiformes clade to which the ticks belong: Opilioacarida (Dunlop and Bernardi, Reference Dunlop and Bernardi2014) and Mesostigmata (Joharchi et al., Reference Joharchi, Vorontsov and Walter2021). Given that most arachnids have a fossil record going back to the Palaeozoic, the relatively young (Cretaceous) age of the oldest parasitiform mites is probably an artefact of a lack of appropriate fossil localities for preserving animals of this nature, given that many modern parasitiforms are soil organisms which are less likely to end up in lacustrine environments where they could be buried by sediment. That said, Burmese amber ticks retain their importance by offering (a) the oldest calibration points to date for molecular phylogenies of several living genera, and (b) for demonstrating that during the mid-Cretaceous the tick fauna of the amber forest included what appear to be both modern and extinct genera living side by side. It may be noted that an undescribed immature tick from Spanish amber would push the oldest tick fossils to 105 Ma (Peñalver et al., Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017).
With almost 250 living species, Ixodes is the most diverse modern tick genus and contains several species of medical importance, such as the paralysis tick I. holocyclus in Australia and the Lyme disease vectors Ixodes ricinus in the Palearctic and Ixodes scapularis in the Nearctic (Padula et al., Reference Padula, Leister and Webster2020; Gilbert, Reference Gilbert2021). Ixodes antiquorum sp. nov. is the oldest record of Ixodes, predating the Baltic amber species (Weidner, Reference Weidner1964) by more than 50 million years. A further putative (non-amber) Ixodes from the Eocene of Wyoming in the USA is not demonstrably a tick (Dunlop, Reference Dunlop2011). A record of the extant species Ixodes sigelos Keirans, Clifford and Corwin, 1976 from a Holocene owl pellet in Argentina (Sanchez et al., Reference Sanchez, Nava, Lareschi, Ortiz and Guglielmone2010) is the only other unequivocal (sub)fossil in this genus. The presence of Ixodes, and its concomitant clade Prostriata, was to be expected in Burmese amber based on the presence of several genera from its sister-group Metastriata. According to current molecular dating, the split between Prostriata and Metastriata probably occurred considerably earlier at ca. 234 ± 18 Ma (Mans et al., Reference Mans, Featherston, Kvas, Pillay, de Klerk, Pienaar, de Castro, Schwan, Lopez, Teel, Pérez de León, Sonenshine, Egekwu, Bakkes, Heyne, Kanduma, Nyangiwe, Bouattour and Latif2019).
Ixodes is a cosmopolitan genus today, occurring on several continents (Clifford et al., Reference Clifford, Sonenshine, Keirans and Kohls1973; Fukunaga et al., Reference Fukunaga, Yabuki, Hamase, Oliver and Nakao2000). As noted above, I. antiquorum sp. nov. appears to be most closely related to modern Australian species. This is interesting for two reasons. First, it has long been recognized that there is a fundamental difference between Australian Ixodes species and all the other Ixodes, such that the Australian taxa cluster together phylogenetically and some authors even questioned the monophyly of the genus (Fukunaga et al., Reference Fukunaga, Yabuki, Hamase, Oliver and Nakao2000; Klompen et al., Reference Klompen, Black, Keirans and Norris2000, Reference Klompen, Lekveishvili and Black2007; Shao et al., Reference Shao, Barker, Mitani, Aoki and Fukunaga2005). Other studies support a monophyletic Ixodes (Charrier et al., Reference Charrier, Hermouet, Hervet, Agoulon, Barker, Heylen, Toty, McCoy, Plantard and Rispe2019), but the fact remains that there is a deep division between the Australian and non-Australian taxa, with molecular dating suggesting a split at 224 ± 18 Ma (Mans et al., Reference Mans, Featherston, Kvas, Pillay, de Klerk, Pienaar, de Castro, Schwan, Lopez, Teel, Pérez de León, Sonenshine, Egekwu, Bakkes, Heyne, Kanduma, Nyangiwe, Bouattour and Latif2019). The fact that the Burmese amber nymph has Australasian affinities is thus interesting in anchoring this lineage to at least 100 Ma; the non-Australian Ixodes lineage is anchored on the Baltic amber fossil to ca. 49 Ma.
Ixodes antiquorum sp. nov. is also of considerable biogeographical interest and supports the hypothesis that the flora and fauna of Burmese amber have, at least in part, Gondwanan origins (Poinar, Reference Poinar2018). We hypothesize that the ancestors of our new species could have originated in Australia and then migrated onto the Burma Terrane about 155 Ma via the Woyla Arc (Hall, Reference Hall2012) (see also above) before rafting north towards Asia on this terrane. The hypothesis that Burmese amber fossils may have Gondwanan affinities is not new and has been previously discussed (Hall, Reference Hall2012; Yamamoto et al., Reference Yamamoto, Caron and Bortoluzzi2019). Possible Gondwanan origins have been proposed for, e.g. several beetles (Kirejtshuk and Poinar, Reference Kirejtshuk and Poinar2006, Reference Kirejtshuk, Poinar and Azar2013; Cognato and Grimaldi, Reference Cognato and Grimaldi2009; Thayer et al., Reference Thayer, Newton and Chatzimanolis2012; Cai and Huang, Reference Cai and Huang2017; Jałoszyński et al., Reference Jałoszyński, Yamamoto and Takahashi2017; Jarzembowski et al., Reference Jarzembowski, Wang and Zheng2017; Cai et al., Reference Cai, Ślipiński, Leschen, Yin, Zhuo and Huang2018, Reference Cai, Lawrence, Yamamoto, Leschen, Newton, Ślipiński, Yin, Huang and Engel2019; Wu et al., Reference Wu, Li and Ding2018; Yamamoto et al., Reference Yamamoto, Caron and Bortoluzzi2019) and bugs (Poinar and Brown, Reference Poinar and Brown2016).
An alternative hypothesis is that the West Burma block rifted from Australia in the Early-Middle Permian (~270 Ma) and was attached to Asia by the upper Triassic (~200 Ma) (Sevastjanova et al., Reference Sevastjanova, Hall, Rittner, Paw, Naing, Alderton and Comfort2016; Metcalfe, Reference Metcalfe2017; Clarke et al., Reference Clarke, Limaye, McKenna and Oberprieler2019). The fossil species found in Burmese amber would, therefore, have been endemic to the Asiatic region by the time of fossilization ~100 Ma later. If the affinities of I. antiquorum sp. nov. to extant Australian Ixodes lineages have a monophyletic origin, this scenario would suggest a much more ancient origin for Ixodes (>270 Ma) and by implication ticks in general. However, it would not explain the extant restriction of Australasian Ixodes since subsequent dispersal on mainland Asia from 200 Ma would have suggested a much wider distribution for the Australasian Ixodes, given that the lineage leading to the Burmese fossils would have survived in Asia for more than 100 million years. Tick fossils may therefore have important implications for hypotheses on the origin and timing of the West Burma block.
The C. burmanicum female together with a feather barb completes the finding of this tick in amber from larvae (Poinar and Brown, Reference Poinar and Brown2003), a nymph with dinosaur feather (Peñalver et al., Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017), and the female from the current study. The barb from a feather found corresponds to a dinosaur feather according to Carroll et al. (Reference Carroll, Chiappe and Bottjer2019). For C. burmanicum, the hooks on the internal side of the third palpal segment in all described stages confirm that these ticks belong to the same species. The finding of two different life stages with dinosaur feathers supports the Peñalver et al. (Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017) hypothesis that this tick was a parasite of the Pennaraptora clade of dinosaurs.
While the presence of fossils referable to extant tick genera in Burmese amber points to a considerable degree of evolutionary stasis in some lineages (Amblyomma, Haemaphysalis and now also Ixodes), the discovery of K. fossus gen. et sp. nov. that combines the features of both hard and soft ticks is of considerable interest and importance. There is precedence for ticks in the mid-Cretaceous having body plans, unlike species that we know today. Deinocroton was placed in an extinct family, Deinocrotonidae (Peñalver et al., Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017), and differs from living species in the ornamentation of its integument, the shape of the palp and the shapes of the preanal and genital grooves. It may be related to the living family Nuttalliellidae with its single species, Nuttalliella namaqua Bedford, Reference Bedford1931, being termed a ‘living fossil’ since it presents intermediate characters between hard and soft ticks (Bedford, Reference Bedford1931; Mans et al., Reference Mans, de Klerk, Pienaar and Latif2011). In detail, it has an argasid-like body, argasid-like feeding behaviour, but an ixodid-like pseudoscutum and a sub-terminal hypostome (Mans et al., Reference Mans, de Klerk, Pienaar, de Castro and Latif2012). Deinocroton also preserves these pseudoscutum and hypostome characters (Peñalver et al., Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017).
Khimaira fossus gen. et sp. nov. is neither a nuttalliellid nor a deinocrotonid. It has a soft, argasid-like body combined with a well-developed, ixodid-like terminal basis capitulum and a scutum. The basis capituli differs completely from the mouthparts of both Deinocrotonidae and Nuttalliellidae which is underdeveloped compared to the Khimairidae. These features, in combination, are so distinct and incongruous with respect to the known families (living and extinct) that we believe the new fossil merits its own family (Khimairidae fam. nov.) since it seems to be a truly chimaeric fusion of a hard and soft tick. This makes it a much likelier candidate than either deinocrotonids or nuttalliellids of being a last common ancestral lineage to the two main living tick families. With regard to its biology, the soft body suggests that both nymphal and adult stages would have exhibited rapid feeding behaviour, as observed for living Argasidae and Nuttalliellidae (Mans et al., Reference Mans, de Castro, Pienaar, de Klerk, Gaven, Genu and Latif2016). By contrast, the terminal gnathosoma would imply that larvae may have undergone prolonged feeding, as observed in some members of Argasidae and all Ixodidae and Nuttalliellidae. This suggests that, as in the Nuttalliellidae, the ancestral biology of ticks is represented by larvae that showed prolonged feeding, with nymphs and adults showing rapid feeding (Mans et al., Reference Mans, de Castro, Pienaar, de Klerk, Gaven, Genu and Latif2016). As such, Khimairidae, like Nuttalliellidae, presents characters shared among argasids and ixodids and may explain the striking differences in the biology of the two main tick families through sub-functionalization after they diverged from one another.
Khimaira fossus gen. et sp. nov. is mid-Cretaceous in age, but the Ixodidae/Argasidae split must predate the Prostriata/Metastriata one (see above), with published mitochondrial gene molecular dates for the origins of the two main families ranging in the literature from the Early to Late Permian: 290 ± 23 to 260 ± 21 Ma (Mans et al., Reference Mans, de Klerk, Pienaar, de Castro and Latif2012, Reference Mans, Featherston, Kvas, Pillay, de Klerk, Pienaar, de Castro, Schwan, Lopez, Teel, Pérez de León, Sonenshine, Egekwu, Bakkes, Heyne, Kanduma, Nyangiwe, Bouattour and Latif2019). In this scheme, all the major tick lineages found in Burmese amber originated well in advance of the formation of the Incertus Arc at 155 Ma. These include Ixodes (224 ± 18 Ma), Amblyomma (144 ± 12 Ma) and Haemaphysalis (173 ± 14 Ma) (Mans et al., Reference Mans, Featherston, Kvas, Pillay, de Klerk, Pienaar, de Castro, Schwan, Lopez, Teel, Pérez de León, Sonenshine, Egekwu, Bakkes, Heyne, Kanduma, Nyangiwe, Bouattour and Latif2019), which would have allowed dispersal and occupation of the Burma terrane by these lineages from Australia. Based on this scenario, Gondwanan lineages that also originated before the formation of the Incertus Arc might also be expected in Burmese amber. This would include Bothriocrotoninae (180 ± 15 Ma) as well as Argasidae (223 ± 20 Ma); the latter potentially supported by the presence of an ornithodorid tick in New Jersey amber (Klompen and Grimaldi, Reference Klompen and Grimaldi2001). The presence of Bothriocrotoninae in Burmese amber would offer particularly strong support for the colonization of the Incertus Arc from Australia. Conversely, the presence of Deinocrotonidae and Khimairidae in Australian amber deposits (if these could be discovered) would be further support of this hypothesis.
A much younger origination date for ticks has been postulated in the Jurassic ~192 ± 50 Ma (Beati and Klompen, Reference Beati and Klompen2019), with the split between Ixodidae and Argasidae at ~178 ± 50 Ma. However, in this molecular dating based on the nuclear 18S rRNA gene, the prostriates originate at ~112 ± 50 Ma, metastriates at ~97 ± 12 Ma, Amblyomminae at ~71 ± 25 Ma and Haemaphysalinae at ~33 ± 25 Ma (Beati and Klompen, Reference Beati and Klompen2019). Given that fossils assignable to Ixodes (this study), Amblyomma and Haemaphysalis have already been found in 100 Ma Burmese amber (Chitimia-Dobler et al., Reference Chitimia-Dobler, Cancian de Araujo, Ruthensteiner, Pfeffer and Dunlop2017, Reference Chitimia-Dobler, Pfeffer and Dunlop2018), the nuclear clock seems to underestimate divergence times for ticks, which suggests in turn that the origin of ticks may actually lie somewhat earlier, perhaps between 273 and 192 Ma (i.e. mid-Permian to Early Jurassic). Again this underscores the importance of Burmese amber tick fossils for our understanding of tick origins and evolution.
Given its relatively young age, our new fossil is unlikely to be directly ancestral to either of the modern families (Fig. 5). Instead, we suspect there was a late Palaeozoic, or perhaps early Mesozoic, lineage from which both Ixodidae and Argasidae evolved and that K. fossus gen. et sp. nov. is part of this group and retained these intermediate character states through into the late Mesozoic. A similar scenario from Burmese amber was observed in the remarkable tailed spider Chimerarachne yingae (Wang et al., Reference Wang, Dunlop, Selden, Garwood, Shear, Müller and Lei2018). This extinct species retains several plesiomorphic character states for spiders (Huang et al., Reference Huang, Hormiga, Cai, Su, Yin, Xia and Giribet2018; Wang et al., Reference Wang, Dunlop, Selden, Garwood, Shear, Müller and Lei2018) most obviously the retention of a flagelliform telson, but cannot be directly ancestral to other Araneae as spiders referable to the extant clade Mesothelae were already present in the Late Carboniferous. We hypothesize that the mid-Cretaceous Burmese amber hosted late survivors of earlier radiations of at least the ticks and spiders among the arachnids, and it would be interesting to see if this is true of any other arachnid groups. Examples of unusual Burmese amber insects with character combinations not seen in living groups are also known (Bai et al., Reference Bai, Beutel, Klass, Zhang, Yang and Wipfler2016; Poinar and Brown, Reference Poinar and Brown2017), and may represent further examples of relict arthropod taxa which maintained a presence until near the end of the Mesozoic – at least on the putative island hosting the Burmese amber forest.

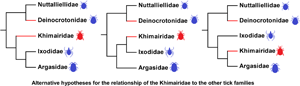
Fig. 5. A representation of the possible systematic relationships among living and extinct tick families adapted from Peñalver et al. (Reference Peñalver, Arillo, Delclòs, Peris, Grimaldi, Anderson, Nascimbene and Pérez-de la Fuente2017) and Mans et al. (Reference Mans, Featherston, Kvas, Pillay, de Klerk, Pienaar, de Castro, Schwan, Lopez, Teel, Pérez de León, Sonenshine, Egekwu, Bakkes, Heyne, Kanduma, Nyangiwe, Bouattour and Latif2019). Extinct lineages are depicted by red branches, while the potential divergence points for the Khimairidae are indicated as sister lineage to the Ixodidae/Argasidae (preferred placement), or the Ixodidae, or the Argasidae.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022000269.
Data
The data reported in this paper are detailed in the main text.
Acknowledgements
We thank Patrick Müller for initially making much of this material available for study. This research was supported using resources of the VetCore Facility (Imaging) of the University of Veterinary Medicine Vienna.
Author contributions
L.C.-D. took the photos and described the species; B.J.M. contributed to the description and interpreted the tick evolution; S.H. made the microCT scanning providing images and videos for the description; J.A.D. made the drawing for all specimens. L.C.-D., B.J.M. and J.A.D. wrote the manuscript. All authors read and proofed the final version of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.