Introduction
Leishmaniasis and cancer
Leishmaniasis is a group of vector-borne diseases caused by intracellular protozoan belonging to the genus Leishmania. Annually, approximately 1.5–2 million new cases are reported worldwide being 310 million people at risk. The mortality rate of the disease varies from 40 000 to 70 000 cases per year (Torres-Guerrero et al., Reference Torres-Guerrero, Quintanilla-Cedillo, Ruiz-Esmenjaud and Arenas2017). Clinical manifestations vary depending on the Leishmania species and the immune status of the host, among others. The clinical symptoms of cutaneous leishmaniasis (CL) are mostly restricted to the skin lesions with diverse appearances, from localized to body extended wounds or mucosal affectations. However, visceral leishmaniasis (VL) is characterized by severe organic symptoms and might lead to death (Den Boer et al., Reference Den Boer, Argaw, Jannin and Alvar2011; Torres-Guerrero et al., Reference Torres-Guerrero, Quintanilla-Cedillo, Ruiz-Esmenjaud and Arenas2017). Malnutrition, acquired immune deficiency syndrome and cancer are important factors that affect the host immune system and lead to more severe clinical symptoms in patients with leishmaniasis (Ezra et al., Reference Ezra, Ochoa and Craft2010; Nweze et al., Reference Nweze, Nweze and Onoja2020).
Cancer is a group of diseases involving an abnormal growth of cells, which tend to proliferate in an uncontrolled way and, in some cases, metastasize. It can affect almost any tissue of the body. After coronary artery diseases, cancer is the second major cause of death in humans. Each year, the global mortality rate of cancers is estimated at 8.2 million deaths and approximately 14.1 million new cases are being reported (Torre et al., Reference Torre, Siegel, Ward and Jemal2016). The clinical manifestations of cancers are wide-ranging and the immunosuppression is a critical side-effect to be considered during the management of cancers (Blagosklonny, Reference Blagosklonny2013).
The possible association of leishmaniasis and malignancies (cancers)
Although smoking is one of the principal causes of cancer development, infections are also a risk factor, mainly those caused by bacteria (Helicobacter pylori) (Nguewa et al., Reference Nguewa, Villa, Notario, Villa and Vinas2016) and viruses (Human papillomavirus, Hepatitis B and C viruses, Herpes virus, Epstein–Bar virus and human T-cell leukaemia-lymphoma virus) (Liao, Reference Liao2006). However, certain parasitic infections (by Opisthorchis, Clonorchis, Theileria and Schistosoma) can also raise the risk of developing some types of cancers and may contribute to the appearance of malignancies which makes them possible models to study host–parasite interactions and mechanisms of cancer (De Martel et al., Reference De Martel, Ferlay, Franceschi, Vignat, Bray, Forman and Plummer2012; Tretina et al., Reference Tretina, Gotia, Mann and Silva2015; Cheeseman and Weitzman, Reference Cheeseman and Weitzman2017).
The association of leishmaniasis and malignancies in human and animal models has been highlighted in previous studies (Kopterides et al., Reference Kopterides, Mourtzoukou, Skopelitis, Tsavaris and Falagas2007; Ferro et al., Reference Ferro, Palmieri, Cavicchioli, Zan, Aresu and Benali2013; Al-Kamel, Reference Al-Kamel2017). Due to the relatively similar clinical manifestations in certain leishmaniasis forms and cancers, misdiagnosis might occur in the clinic (Toogeh et al., Reference Toogeh, Shirkoohi, Nickbin, Najafi, Salimi, Farsi and Ferdowsi2010; Schwing et al., Reference Schwing, Pomares, Majoor, Boyer, Marty and Michel2019). For instance, the diagnosis of childhood leukaemia should be carefully differentiated from VL, especially in endemic areas where the concurrent occurrence had been reported (Vasconcelos et al., Reference Vasconcelos, Azevedo-Silva, Thuler, Pina, Souza, Calabrese and Pombo-de-Oliveira2014). Similarly, cutaneous and mucocutaneous leishmaniasis may be clinically misdiagnosed as squamous cell carcinoma (SCC) (Ramos et al., Reference Ramos, Munez, García-Domínguez, Martinez-Ruiz, Chicharro, Banos, Suarez-Massa and Cuervas-Mons2015; Oetken et al., Reference Oetken, Hiscox, Orengo and Rosen2017). These data point out the possible similar association in clinical manifestations of leishmaniasis and tumoural alterations. Moreover, failures at epigenetic level to maintain integrity of chromosomes is one contributing factor in cancer and Leishmania parasites also modulate and destabilize the host chromatin structure leading to potential changes in relevant immune-related genes and responses (Sarkar et al., Reference Sarkar, Leung, Baguley, Finlay and Askarian-Amiri2015; Afrin et al., Reference Afrin, Khan and Hemeg2019; Dacher et al., Reference Dacher, Tachiwana, Horikoshi, Kujirai, Taguchi, Kimura and Kurumizaka2019).
In addition, numerous compounds with anti-tumour activity have exhibited potent leishmanicidal properties (Table 1). The use of common drugs for the treatment of leishmaniasis and cancer might further propose and highlight the presence of plausible similarities in their molecular mechanisms of action, immunopathobiology and therapeutic targets in both diseases (Table 2) (Kopterides et al., Reference Kopterides, Mourtzoukou, Skopelitis, Tsavaris and Falagas2007; Miguel et al., Reference Miguel, Yokoyama-Yasunaka, Andreoli, Mortara and Uliana2007, Reference Miguel, Yokoyama-Yasunaka and Uliana2008; Moulisha et al., Reference Moulisha, Kumar and Kanti2010; Toogeh et al., Reference Toogeh, Shirkoohi, Nickbin, Najafi, Salimi, Farsi and Ferdowsi2010). Furthermore, chemotherapies administered against some forms of cancer display immune dysfunctions and/or immunosuppression. An example is bortezomib, a proteasome inhibitor which decreases the dendritic cells’ (DCs) activity, the number of T lymphocytes and interferon (IFN) gamma production (Nucci and Anaissie, Reference Nucci and Anaissie2009). This immunosuppressive effect may lead to leishmaniasis development as an opportunistic infection in antitumour-treated patients but also in immunocompetent ones leading to similar symptoms (Piro et al., Reference Piro, Kropp, Cantaffa, Lamberti, Carillio and Molica2012; Cencini et al., Reference Cencini, Lazzi and Fabbri2015; Torti et al., Reference Torti, Pulini, Morelli, Bacci and Di Bartolomeo2015; Schwing et al., Reference Schwing, Pomares, Majoor, Boyer, Marty and Michel2019). Additionally, a synergistic relationship between Leishmania parasites and cancer cells has been highlighted (Morsy, Reference Morsy2013). Now, case reports and clinical observations suggest that leishmaniasis may be a risk factor for certain cancers and that cancer immunosuppression may facilitate Leishmania infections (Morsy, Reference Morsy2013; Liao et al., Reference Liao, Jin, Yu and Jiang2018; Nicolas et al., Reference Nicolas, Elliott Koury, Salibi, Nehme, Mitri, El Sayegh, Rached and Khoury2018; Carrillo-Larco et al., Reference Carrillo-Larco, Acevedo-Rodriguez, Altez-Fernandez, Ortiz-Acha and Ugarte-Gil2019; Claudio et al., Reference Claudio, Alessandro, Luca, Jacopo and Katia2019). However, a comprehensive review regarding common expressed proteins in Leishmania parasites and cancer cells is lacking. Therefore, reviewing and highlighting such functional proteins might reveal valuable information regarding the possible shared mechanisms of pathogenicity and possible therapeutic targets in leishmaniasis and cancers in the future.
Table 1. Some common compounds used against cancer and leishmaniasis
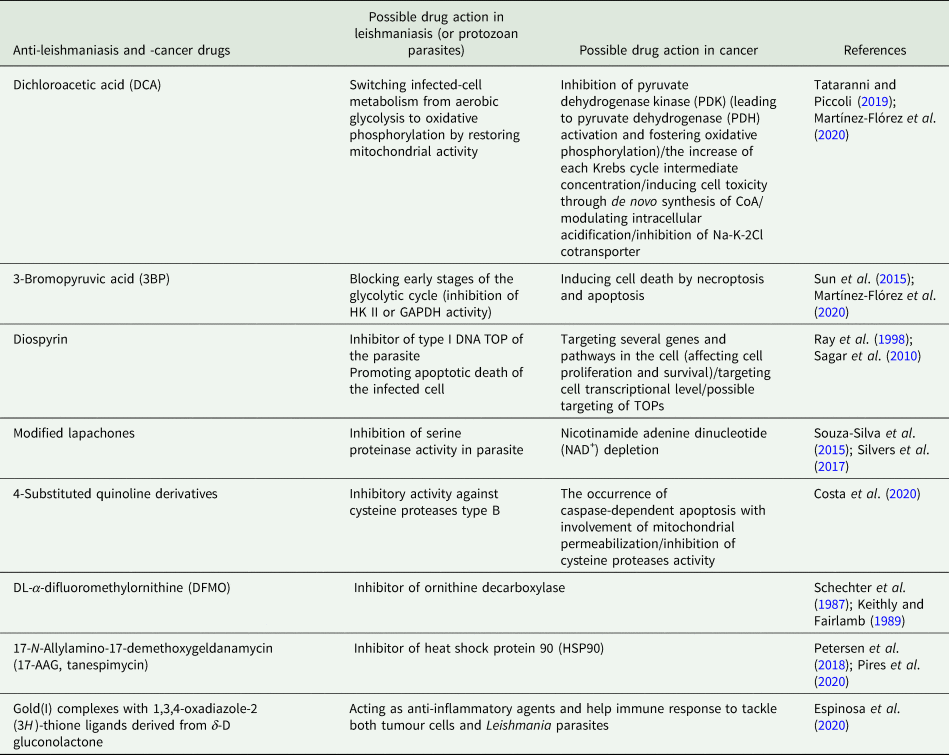
Table 2. Possible functions of several common proteins expressed in Leishmania parasites and cancer cells and plausible inhibitors/drugs against such proteins
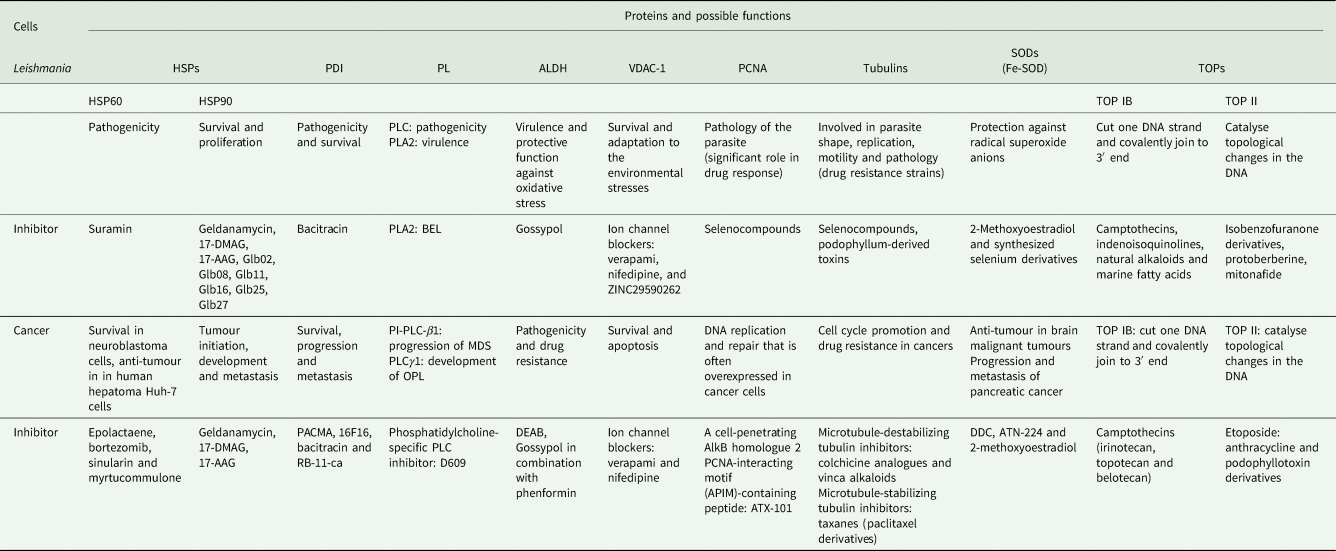
Drugs for the treatment of leishmaniasis and cancer with common signalling processes
Drug repurposing is an extensively strategy used to identify new microbicidal compounds including leishmanicidal agents. The effect of antitumour chemical compounds in leishmaniasis treatment might suggest the existence of possible similar mechanisms of pathogenicity and common therapeutic targets in Leishmania parasites and cancer cells. Selenocompounds have demonstrated antitumour properties blocking the mammalian target of rapamycin (mTOR) pathway (Ibanez et al., Reference Ibanez, Agliano, Prior, Nguewa, Redrado, Gonzalez-Zubeldia, Plano, Palop, Sanmartin and Calvo2012) and reduced Leishmania parasite burden during ‘in vitro’ assays. Furthermore, these compounds were able to reduce the expression of leishmanial genes involved in cell cycle, resistance to treatment and virulence at the mRNA level (Fernández-Rubio et al., Reference Fernández-Rubio, Campbell, Vacas, Ibañez, Moreno, Espuelas, Calvo, Palop, Plano and Sanmartin2015). Similarly, naphtilamide derivatives which were previously synthesized as antitumour agents (Karelia et al., Reference Karelia, Sk, Singh, Gowda, Pandey, Ramisetti, Amin and Sharma2017), decreased intracellular amastigotes burden, caused cell cycle arrest and diminished the topoisomerase-2 (TOP II), mini-chromosome maintenance complex (MCM4) and proliferating cell nuclear antigen (PCNA) mRNA levels (Fernández-Rubio et al., Reference Fernández-Rubio, Larrea, Guerrero, Herrero, Gamboa, Berrio, Plano, Amin, Sharma and Nguewa2019). Anti-cancer and anti-leishmaniasis effects of herbal compounds such as pentacyclic triterpenoid are well known (Moulisha et al., Reference Moulisha, Kumar and Kanti2010). Fatty acids from natural sources are inhibitors of therapeutic targets in cancer cells and Leishmania parasites (Carballeira et al., Reference Carballeira, Montano, Cintrón, Márquez, Rubio, Prada and Balaña-Fouce2011, Reference Carballeira, Morales-Guzman, Alvarez-Benedicto, Torres-Martinez, Delgado, Griebenow, Tinoco, Reguera, Perez-Pertejo and Carbajo-Andres2018; Carballeira, Reference Carballeira2013).
Moreover, a set of compounds described as antitumoural drugs also exhibited leishmanicidal activities. Alkylating antineoplastic agents such as cisplatin are inductors of cell death in both, tumour cells and parasites (Fuertes et al., Reference Fuertes, Alonso and Pérez2003; Nguewa et al., Reference Nguewa, Fuertes, Iborra, Najajreh, Gibson, Martínez, Alonso and Pérez2005). Miltefosine (hexadecylphosphocholine, HePC), an alkyl phospholipids compound, has been originally considered for breast cancer and other solid tumours’ treatment. Two compounds of the alkylphosphocholine group, octadecyl-phosphocholine and hexadecylphosphocholine-miltefosine (HePC), have been found to have antineoplastic activity. The mechanism of antitumour action of these compounds was involved in the inhibition of substrate phosphorylation by protein kinase C (PKC) [triggering programmed cell death (apoptosis)]. The presence of PKC on Leishmania membrane led to the further investigations on such compounds against CL. It has been indicated that miltefosine inhibits the biosynthesis of the glycosyl phosphatidyl inositol receptor, a vital molecule for Leishmania intracellular survival. Moreover, this compound interferes with the synthesis of leishmanial-phospholipase (PL) and PKC. The metabolic action of miltefosine affects the biosynthesis of glycolipids and membrane glycoproteins of the Leishmania parasite, leading to apoptosis (Sundar and Olliaro, Reference Sundar and Olliaro2007; Perez et al., Reference Perez, Fuertes, Nguewa, Castilla and Alonso2008; dos Santos Nogueira et al., Reference dos Santos Nogueira, Avino, Galvis-Ovallos, Pereira-Chioccola, Moreira, Romariz, Molla and Menz2019; Fernández-Rubio et al., Reference Fernández-Rubio, Larrea, Guerrero, Herrero, Gamboa, Berrio, Plano, Amin, Sharma and Nguewa2019). Another example is tamoxifen (as a triphenylethylene), a breast cancer drug that has shown an appropriate efficacy in the treatment of leishmaniasis (Miguel et al., Reference Miguel, Yokoyama-Yasunaka, Andreoli, Mortara and Uliana2007, Reference Miguel, Yokoyama-Yasunaka and Uliana2008). Due to the activity of tamoxifen as an oestrogen receptor modulator, this drug has been used in the prevention and treatment of breast cancer. However, it has been elucidated that many biological effects of tamoxifen are independent of the oestrogen machinery, including modulation of calmodulin, kinases and caspases, inhibition of the acidification of intracellular organelles, interference in ceramide metabolism, and partitioning into lipids where it exerts membrane fluidizing and antioxidant activities. Oestrogen receptor-modulated responses are not present in Leishmania parasites. On the other hand, tamoxifen is able to inhibit the acidification of organelles in different cell types in an oestrogen-independent pathway. It seems that tamoxifen modifies the intravacuolar pH of Leishmania-infected macrophages inducing a condition where the drug activity against the Leishmania is increased (Miguel et al., Reference Miguel, Yokoyama-Yasunaka, Andreoli, Mortara and Uliana2007, Reference Miguel, Yokoyama-Yasunaka and Uliana2008). In addition, camptothecin and its analogues target topoisomerase IB (TOP IB) and inhibit in Leishmania the activity of this relaxing enzyme for supercoiled DNA. Similarly, indenoisoquinolines, which are also TOP IB poisons, initially developed as antitumour compounds (Antony et al., Reference Antony, Kohlhagen, Agama, Jayaraman, Cao, Durrani, Rustum, Cushman and Pommier2005), are able to decrease parasite burden ‘in vitro’ and ‘in vivo’ in a mouse model (Balaña-Fouce et al., Reference Balaña-Fouce, Prada, Requena, Cushman, Pommier, Álvarez-Velilla, Escudero-Martínez, Calvo-Álvarez, Pérez-Pertejo and Reguera2012). Furthermore, quinone derivatives that act as antitumour agents, showed anti-leishmanial activity through different mechanisms of action, including TOP II and trypanothione reductase inhibition (Sett et al., Reference Sett, Basu, Ghosh and Das1992; Mukherjee et al., Reference Mukherjee, Das, Kole, Karmakar, Datta and Das2004; Singh and Dey, Reference Singh and Dey2007; Shukla et al., Reference Shukla, Patra and Dubey2011).
Immunological coincidences between leishmaniasis and cancer
Investigations have indicated the effect of Leishmania parasites on the immune system of patients with cancer thus triggering the modulation of anti-cancer immunity. In 2011, Kumar et al. highlighted the role of Leishmania in mutual modulation of the immune system in a patient with Hodgkin's lymphoma (Kumar et al., Reference Kumar, Daga, Kamble, Sothwal, Singh, Nayak and Raizada2011). It's clear that in both diseases the host immune response is critical for the disease outcome. In this sense, immune checkpoints are essential for the regulation of the immune system homoeostasis and metastasis in cancer (Safarzadeh et al., Reference Safarzadeh, Alizadeh, Beyranvand, Jozaaee, Hajiasgharzadeh, Baghbanzadeh, Derakhshani, Argentiero, Baradaran and Silvestris2020). These are also important factors regulating the function of T-cells and can be differentially modulated during pathogen infections (Cai et al., Reference Cai, Liu, Zhong, Zheng, Xiao, Li, Song, Li, Xu and Wu2020). Leishmaniasis shares several key immunoregulatory features with cancer. Thus, in some forms of leishmaniasis, a number of important immune checkpoint molecules have also been identified (Kumar et al., Reference Kumar, Chauhan, Ng, Sundar and Engwerda2017). Cytotoxic T-lymphocyte-associated protein 4 is one of the differentially induced immune checkpoints upon infection that may be targeted to ameliorate disease progression (Viana et al., Reference Viana, Magalhães, Giunchetti, Dutra and Gollob2019). These authors state that some Leishmania species induce a more inflammatory profile which also is well-established in cancer progression. Pattern recognition receptor expression and activation have pro-inflammatory effects on the tumour microenvironment via Toll-like receptor (TLR) signalling (McCall et al., Reference McCall, Muccioli and Benencia2020). Leishmania infections have differential capacities to activate TLRs and are able to block the TLR-based pro-inflammatory downstream signals. Parasite-derived microvesicles can activate specific TLR-based downstream immune inhibitory signals via CD200 to evade macrophage defenses and favour infection (Saha et al., Reference Saha, Basu, Guin, Gupta, Mitterstiller, Weiss, Jana and Ukil2019; Sauter et al., Reference Sauter, Madrid, de Assis, Sá-Nunes, Torrecilhas, Staquicini, Pasqualini, Arap and Cortez2019).
Another shared mechanism is type I IFN-driven immunity. IFNs play essential roles in context-specific anti-pathogen responses and act in many immune-related processes in cancer including therapy (Silva-Barrios and Stager, Reference Silva-Barrios and Stager2017; Sprooten et al., Reference Sprooten, Agostinis and Garg2019). Recently, it has been discovered that type I IFNs are involved in the negative regulation of CD4+ T cell responses in patients with VL in order to thus promote their persistence by suppressing anti-parasitic immunity (Kumar et al., Reference Kumar, Bunn, Singh, Ng, de Oca, Rivera, Chauhan, Singh, Faleiro and Edwards2020). It seems that the action of IFNs may be by targeting DCs to suppress consequently priming and/or expansion of Th1 specific cells during the infection. These authors have also stated with animal models the potential of targeting type I IFN signalling to strength protective immunity. In this regard, some molecules of Leishmania major are able to affect DC maturation thus potentially enhancing DC-based vaccines for cancer (Arab et al., Reference Arab, Motamedi and Hadjati2019). Furthermore, live attenuated nonpathogenic Leishmania parasites directly may induce immune-stimulant responses to regress breast cancer grown (Caner et al., Reference Caner, Sadıqova, Erdoğan, Namlıses, Nalbantsoy, Oltulu, Toz, Yiğittürk, Ozkök and Gunduz2020). Interestingly, these attenuated parasites led to a higher percentage of CD4+ and CD8+ T-cells secreting pro-inflammatory cytokines tumour necrosis factor-α (TNF-α), interleukin-12 (IL-12), IFN-γ, inducible nitric oxide synthase and IL-2, and finally, inducing tumour cell death. Cancer therapy is an essential research area and protein kinases are major targets for drugs. The mTOR is a highly conserved serine/threonine protein kinase that is central regulating essential cellular processes and mTOR inhibitors are used in cancer therapy (Chen and Zhou, Reference Chen and Zhou2020). Leishmania infection activates this key host protein kinase pathway via its phosphorylation which have important effect towards host cellular physiology (M2 macrophage phenotype polarization) and parasite survival inside macrophages through down-regulation of the nuclear factor-κB and signal transducer and activator of transcription 3 oncogene factors (Kumar et al., Reference Kumar, Das, Mandal, Verma, Abhishek, Kumar, Kumar, Ghosh and Das2018). Another type of immune-related molecules which regulate diverse cellular processes involved in cancer and leishmaniasis are Ras, small cellular GTPases. Ras mutations are frequently observed in cancers and leukaemia leading to different isoforms. Currently, research data demonstrate that these isoforms are differentially activated by CD40 to modulate effector signals like inflammation playing crucial roles in infectious diseases and tumour regression between others (Nair et al., Reference Nair, Chakraborty, Banerji, Srivastava, Navare and Saha2020). Leishmania infections are able to inhibit CD40-induced N-Ras activation as a survival strategy switching CD40 signalling leading to inhibition of the p38MAPK pathway, the master regulator of transcript stability and tumour progression (Chakraborty et al., Reference Chakraborty, Srivastava, Jha, Nair, Pandey, Srivastava, Kumari, Singh, Krishnasastry and Saha2015; Soni et al., Reference Soni, Anand and Padwad2019).
From potential targets to drug repurposing
Novel shared targets may be identified by analysing proteins that play key roles in each disease. To date, mass spectrometry and functional proteomics along with other integrative-omics and multi-platforms allow shedding some light on the role of these molecules in different diseases (Cowell and Winzeler, Reference Cowell and Winzeler2019; Khan et al., Reference Khan, Ince-Dunn, Suomalainen and Elo2020; Syu et al., Reference Syu, Dunn and Zhu2020). In addition, some biomarkers of diseases seem to have high potential as drug targets, opening avenue for therapeutic screenings (Sharma et al., Reference Sharma, Garg, Sharma, Capalash and Singh2019; Roy et al., Reference Roy, Shrivastava, Srivastav, Shankar and Srivastava2020). Accordingly, we therefore present some functional proteins that may be useful as shared/interchangeable therapeutic targets for the development pipeline of repurposed drugs against leishmaniasis and cancer.
Protein disulphide isomerases (PDIs)
PDIs belong to a group of multifunctional proteins which are located in the endoplasmic reticulum and catalyse the formation of disulphide bonds during protein creation (Ben Khalaf et al., Reference Ben Khalaf, Muylder, Louzir, McKerrow and Chenik2012). In Leishmania parasites, it has been reported the role of these proteins in pathogenicity and survival. Moreover, their potential immunostimulatory property suggests such proteins as possible drug and vaccine targets against leishmaniasis (Achour et al., Reference Achour, Chenik, Louzir and Dellagi2002; Gupta et al., Reference Gupta, Sisodia, Sinha, Hajela, Naik, Shasany and Dube2007; Ben Khalaf et al., Reference Ben Khalaf, Muylder, Louzir, McKerrow and Chenik2012; Jaiswal et al., Reference Jaiswal, Khare, Joshi, Kushawaha, Sundar and Dube2014; Amit et al., Reference Amit, Dikhit, Singh, Kumar, Suman, Singh, Kumar, Thakur, Das and Das2017).
On the other hand, the expression of PDIs can affect the survival, progression and metastasis of cancer. In fact, PDI gene is up-regulated in different cancer types such as lymphoma, brain, ovarian or kidney among others (Xu et al., Reference Xu, Sankar and Neamati2014). PDI inhibitor developments from natural and synthetic compounds have demonstrated their effectiveness and cytotoxicity against tumours and Leishmania parasites (Ben Khalaf et al., Reference Ben Khalaf, Muylder, Louzir, McKerrow and Chenik2012; Xu et al., Reference Xu, Sankar and Neamati2014; Lee, Reference Lee2017). For instance, propynoic acid carbamoyl methyl amides (PACMA) 31, a new irreversible PDI inhibitor, and 16F16, showed significant anticancer activity in in vitro and in vivo ovarian cancer models (Xu et al., Reference Xu, Butkevich, Yamada, Zhou, Debnath, Duncan, Zandi, Petasis and Neamati2012, Reference Xu, Sankar and Neamati2014). The terminal propynoic group of PACMA covalently reacts with the thiol groups of the active-site cysteines in PDI. This interaction also changes the secondary protein structure of PDI. RB-11-ca, arsenic-containing compounds, sulphhydryl reagents, juniferdin and its analogues, quercetin-3-rutinoside, and bacitracin have been identified as possible PDI inhibitors against cancer cells (Xu et al., Reference Xu, Sankar and Neamati2014). Among PDI inhibitor identified against cancer cells, bacitracin inhibited in vitro promastigote growth as well as amastigote propagation inside macrophages with EC50 values of 39 μ m. This compound blocked both reductase and isomerase activities of PDI in Leishmania parasite (Ben Khalaf et al., Reference Ben Khalaf, Muylder, Louzir, McKerrow and Chenik2012).
Superoxide dismutases (SODs)
SODs are considered potential cellular antioxidants in different cells since they are metalloproteins involved in the breakdown of potentially harmful oxygen molecules, preventing tissue damage. For instance, Fe-SOD protects Leishmania against radical superoxide anions using iron as a cofactor (Opperdoes and Szikora, Reference Opperdoes and Szikora2006; Van Assche et al., Reference Van Assche, Deschacht, da Luz, Maes and Cos2011). SODs are proteins encoded by conserved genes in Leishmania parasites and due to their protective function, are considered possible therapeutic and vaccine targets against leishmaniasis (Paramchuk et al., Reference Paramchuk, Ismail, Bhatia and Gedamu1997; Danesh-Bahreini et al., Reference Danesh-Bahreini, Shokri, Samiei, Kamali-Sarvestani, Barzegar-Jalali and Mohammadi-Samani2011; Sanchez-Moreno et al., Reference Sanchez-Moreno, Gomez-Contreras, Navarro, Marin, Ramírez-Macías, Rosales, Campayo, Cano, Sanz and Yunta2015; Martin-Montes et al., Reference Martín-Montes, Plano, Martín-Escolano, Alcolea, Díaz, Pérez-Silanes, Espuelas, Moreno, Marín and Gutiérrez-Sánchez2017; Rashidi et al., Reference Rashidi, Nguewa, Mojtahedi, Shahriari, Kalantar and Hatam2020a).
Alternatively, the deregulation of the redox homoeostasis is implicated in several diseases, among them malignancies. It had been shown that the activity of Zn-SOD, Mn-SOD and Cu-SOD is decreased in cancer cells. Furthermore, in most brain malignant tumours, apoptosis occurs due to the expression of SODs (Younus, Reference Younus2018) and are proteins inducing the progression and metastasis of pancreatic cancer cells (Oberley and Buettner, Reference Oberley and Buettner1979). Due to the multifunctional role of these proteins, several inhibitors have been developed (Wood et al., Reference Wood, Leese, Leblond, Woo, Ganeshapillai, Purohit, Reed, Potter and Packham2001; Dumay et al., Reference Dumay, Rincheval, Trotot, Mignotte and Vayssière2006; Glasauer et al., Reference Glasauer, Sena, Diebold, Mazar and Chandel2014). For instance, diethyldithiocarbamate (DDC) has been known as an inhibitor of Cu- and Zn-SODs. DDC has antagonistic effects on apoptosis by triggering cytochrome c release and caspase inhibition (Dumay et al., Reference Dumay, Rincheval, Trotot, Mignotte and Vayssière2006). In addition, it has been indicated that SOD1 by the small molecule ATN-224 induced cell death in various non-small-cell lung cancers cells (NSCLCs). ATN-224-dependent SOD1 inhibition enhanced superoxide, which decreased the enzyme activity of the antioxidant glutathione peroxidase, causing an increase in intracellular hydrogen peroxide (H2O2) levels (Glasauer et al., Reference Glasauer, Sena, Diebold, Mazar and Chandel2014). 2-Methoxyoestradiol, a naturally occurring metabolic product of 17-beta-oestradiol, is able to inhibit tubulin polymerization and possesses growth inhibitory and cytotoxic activity in vitro and in vivo. 2-Methoxyoestradiol also inhibited SOD in a tetrazolium salt-based enzyme assay, proposed that oestrogen derivatives could be useful starting points for the development of non-toxic, effective enzyme inhibitors (Wood et al., Reference Wood, Leese, Leblond, Woo, Ganeshapillai, Purohit, Reed, Potter and Packham2001). In addition to application in cancer therapy, due to the expression of SODs in Leishmania parasites, the SOD inhibitory property of this compound can be evaluated against these parasites. In 2017, a series of synthesized selenium derivatives showed in vitro leishmanicidal activities against intracellular amastigote and promastigotes forms of Leishmania braziliensis and Leishmania infantum with significant low toxicity on the parasite-infected-macrophages. Surprisingly, the most active selenium compounds were potent inhibitors of Fe-SOD in both parasite species (Martín-Montes et al., Reference Martín-Montes, Plano, Martín-Escolano, Alcolea, Díaz, Pérez-Silanes, Espuelas, Moreno, Marín and Gutiérrez-Sánchez2017). Moreover, since tubulins are expressed in Leishmania parasites and considered appropriate drug targets, the tubulin polymerization inhibitory property of 2-methoxyoestradiol, is another factor that might underline this compound as a possible drug against leishmaniasis (Morgan et al., Reference Morgan, Ahn, Nzimiro, Fotie, Phelps, Cotrill, Yakovich, Sackett, Dalton and Werbovetz2008).
Phospholipase
PLC facilitates the evasion of protozoan parasites from parasitophorous vacuoles and helps to hydrolyse miltefosine in Leishmania parasites. Such critical functions highlight the role of PLC in the pathogenicity of Leishmania parasites (Breiser et al., Reference Breiser, Kim, Fleer, Damenz, Drube, Berger, Nagel, Eibl and Unger1987; Moudy et al., Reference Moudy, Manning and Beckers2001; Dorlo et al., Reference Dorlo, Eggelte, de Vries and Beijnen2012; Rashidi et al., Reference Rashidi, Nguewa, Mojtahedi, Shahriari, Kalantar and Hatam2020a). Other PLs such as PLA2 play major roles in Leishmania parasites virulence and maintenance in vertebrate hosts. It has been indicated that the use of PLA2 inhibitor such as bromoenol lactone (BEL) led to the reduction of lesions size and decreased the load of parasites in skin in the L. amazonensis-infected BALB/c mice. However, the use of such an inhibitor also induced hepatotoxicity in BALB/c mice (Bordon et al., Reference Bordon, Laurenti, Ribeiro, Toyama, Toyama and Passero2018).
PLCs as intermediate signalling factors for epidermal growth factor and ILs conduct regulatory functions in the immunology of cancers. However, the role of these proteins in the evasion of cancer cells from the host immune system remains unknown (Ramazzotti et al., Reference Ramazzotti, Faenza, Follo, Fiume, Piazzi, Giardino, Fini and Cocco2011). The PLC-isoenzyme profile has not been investigated in Leishmania parasites so far. Nevertheless, the different forms of PLC-isoenzymes including PLC-α, PLC-β1, PLC-ε and PLC-γ1 have been characterized in breast cancer (Cai et al., Reference Cai, Sun, Resaul, Shi, Jiang, Satherley, Davies, Ruge, Douglas-Jones and Jiang2017). It has been shown that nuclear phosphoinositide (PI)-PLC-β1 has a role in the generation, progression and resistance to apoptosis of the cancer cells in patients with myelodysplastic syndromes (MDS) (Ramazzotti et al., Reference Ramazzotti, Faenza, Follo, Fiume, Piazzi, Giardino, Fini and Cocco2011). Other results highlighting the up-regulation of PLC-γ1 in the oral potentially malignant lesion (OPL) were correlated with the development of oral cancer (Ma et al., Reference Ma, Zhou, He and Jiang2013). It was demonstrated that PLC-γ is an important marker in the pathogenicity of cancers (Lattanzio et al., Reference Lattanzio, Piantelli and Falasca2013). The identification of PLC-isoenzymes in Leishmania parasites and the elucidation of possible functions of each PLC-isoenzyme regarding the pathogenicity and clinical manifestations of leishmaniasis might become a new approach for the development of leishmaniasis treatment. It has been shown that inhibition of phosphatidylcholine-specific PLC using tricyclodecan-9-yl-potassium xanthate (D609) selectively targeted proliferation and survival of tumour initiating cells in SCC and ovarian cancer cells. This compound prevented cancer cells from entering the S-phase under growth-factor stimulation without cell death induction (Amtmann and Sauer, Reference Amtmann and Sauer1990; Spadaro et al., Reference Spadaro, Ramoni, Mezzanzanica, Miotti, Alberti, Cecchetti, Iorio, Dolo, Canevari and Podo2008; Iorio et al., Reference Iorio, Ricci, Bagnoli, Pisanu, Castellano, Di Vito, Venturini, Glunde, Bhujwalla and Mezzanzanica2010; Cecchetti et al., Reference Cecchetti, Bortolomai, Ferri, Mercurio, Canevari, Podo, Miotti and Iorio2015). Other compounds including aurintricarboxylic acid, 3013, 3017 and U73122 have been also identified as other possible PLC modulators (Bleasdale et al., Reference Bleasdale, Thakur, Gremban, Bundy, Fitzpatrick, Smith and Bunting1990; Huang et al., Reference Huang, Barrett, Hajicek, Hicks, Harden, Sondek and Zhang2013). These compounds can also be evaluated as inhibitors against PLCs in Leishmania parasites and cancer cells.
Tyrosyl-DNA-phosphodiesterase-1 (TDP-1)
TDP-1 is a PL D able to cleave the phosphodiester bond formed between the tyrosine residue of type I TOP and the 3′ phosphate of DNA. TDP-1 is involved in repairing TOP I–DNA complexes stabilized by TOP IB poisons and performs its activity by hydrolysis of the phosphodiester bond (Banerjee et al., Reference Banerjee, Roy, Sen and Majumder2010). TDP-1 has been firstly described in Leishmania donovani. Recently, indenoisoquinoline derivative with dual TOP IB/TDP-1 inhibitory capability has been tested against L. infantum (Gutiérrez-Corbo et al., Reference Gutiérrez-Corbo, Álvarez-Velilla, Reguera, García-Estrada, Cushman, Balaña-Fouce and Pérez-Pertejo2019).
It has been reported the altered expression of TDP-1 in several cancers (Liu et al., Reference Liu, Zhou, Begum, Sidransky, Westra, Brock and Califano2007; Dean et al., Reference Dean, Fam, An, Choi, Shimizu, Jones, Boerkoel, Interthal and Pfeifer2014; Meisenberg et al., Reference Meisenberg, Gilbert, Chalmers, Haley, Gollins, Ward and El-Khamisy2015). Moreover, single nucleotide polymorphisms are associated with poor survival among small cell lung cancer patients (Lohavanichbutr et al., Reference Lohavanichbutr, Sakoda, Amos, Arnold, Christiani, Davies, Field, Haura, Hung and Kohno2017). Due to its repair action mechanism, TDP-1 is related to resistance to TOP I inhibitors during cancer treatments. Therefore, its status in tumours might predict the effectiveness of the TOP I inhibitors used against cancers. TDP-1 constitutes a promising target in cancer treatment; therefore, the development of inhibitors may be useful to improve the efficacy of chemotherapy (Dean et al., Reference Dean, Fam, An, Choi, Shimizu, Jones, Boerkoel, Interthal and Pfeifer2014; Mozhaitsev et al., Reference Mozhaitsev, Zakharenko, Suslov, Korchagina, Zakharova, Vasil'eva, Chepanova, Black, Patel and Chand2019; Khomenko et al., Reference Khomenko, Zakharenko, Chepanova, Ilina, Zakharova, Kaledin, Nikolin, Popova, Korchagina and Reynisson2020; Mamontova et al., Reference Mamontova, Zakharenko, Zakharova, Dyrkheeva, Volcho, Reynisson, Arabshahi, Salakhutdinov and Lavrik2020).
HSP60
HSPs categorize as a group of proteins that are regulated by different cells in response to exposure to stressful conditions. Several members of these proteins exert chaperone functions by facilitating to refold proteins that were destructed or damaged by the cell stress or by stabilizing new proteins to provide correct folding (Dubey et al., Reference Dubey, Prajapati, Swamy and Pachauri2015). The immunostimulatory property of HSP60 and its up-regulation in Leishmania-infected cells and drug-resistant Leishmania strains lead to consider HSP60 as a valuable biomarker in the vaccination design and the treatment of leishmaniasis (Brandau et al., Reference Brandau, Dresel and Clos1995; Celeste et al., Reference Celeste, Angel, Castro, Gidlund and Goto2004; Requena et al., Reference Requena, Montalvo and Fraga2015; Rashidi et al., Reference Rashidi, Mojtahedi, Shahriari, Kalantar, Ghalamfarsa, Mohebali and Hatam2019). During cancer development, HSP60 is overexpressed in advanced breast and serous ovarian cancers (Desmetz et al., Reference Desmetz, Bibeau, Boissiere, Bellet, Rouanet, Maudelonde, Mangé and Solassol2008; Hjerpe et al., Reference Hjerpe, Egyhazi, Carlson, Stolt, Schedvins, Johansson, Shoshan and Avall-Lundqvist2013). The expression of this protective protein leads to the angiogenesis, metastasis and survival of the cancer cells. Mostly, HSP60 exerts its functions by attaching other proteins. For instance, HSP60 promotes neuroblastoma cells survival through clusterin protein inhibition by a linkage to this protein. In addition, HSP60 binds to the β-catenin and induces metastasis in some cancer cells (Tsai et al., Reference Tsai, Yang, Huang, Chang, Chen, Liu, Teng and Wu2009; Chaiwatanasirikul and Sala, Reference Chaiwatanasirikul and Sala2011). The regulation of apoptosis due to the interaction of HSP60 and cyclophilin D in mitochondrion suggests the dual function of HSP60 in cancer cells (Ghosh et al., Reference Ghosh, Siegelin, Dohi and Altieri2010). In human hepatoma Huh-7 cells, through the interaction of HSP60 with the hepatitis C virus, core proteins induce the production of reactive oxygen species (ROS) and increase the apoptosis which is mediated by TNF-α (Sherman and Multhoff, Reference Sherman and Multhoff2007; Kang et al., Reference Kang, Kim, Kim, Lee, Kim, Lee, Choi and Oh2009). Based on the importance of this chaperone in the viability of both Leishmania and cancer cells, and on the existence of inhibitors targeting them (Cappello et al., Reference Cappello, Marino Gammazza, Palumbo Piccionello, Campanella, Pace, Conway de Macario and Macario2014; Stevens et al., Reference Stevens, Abdeen, Salim, Ray, Washburn, Chitre, Sivinski, Park, Hoang and Chapman2019), HSP60 might be consider a promising therapeutic target against these pathologies. Three known antibiotics (suramin, rafoxanide and closantel) and epolactaene and myrtucommulone have been identified as inhibitors of human HSP60 chaperonin (Meng et al., Reference Meng, Li and Xiao2018; Stevens et al., Reference Stevens, Abdeen, Salim, Ray, Washburn, Chitre, Sivinski, Park, Hoang and Chapman2019). The use of suramin, as first-line chemotherapeutic agent, against Trypanosoma brucei rhodesiense and T. brucei gambiense, might suggest the evaluation of this HSP60 inhibitor against leishmanial-HSP60 (Abdeen et al., Reference Abdeen, Salim, Mammadova, Summers, Goldsmith-Pestana, McMahon-Pratt, Schultz, Horwich, Chapman and Johnson2016; Zininga and Shonhai, Reference Zininga and Shonhai2019). Although the exact mechanism action of this compound remains unknown, probably inhibits some glycolytic enzymes (Willson et al., Reference Willson, Callens, Kuntz, Perié and Opperdoes1993; Zininga and Shonhai, Reference Zininga and Shonhai2019). Sinularin, a compound extracted from the coral Sinularia flexibilis, is able to inhibit HSP60 in melanoma cell-A2058 (Su et al., Reference Su, Lin, Chiu, Chen, Su, Cheng, Hwang, Huang and Wu2012). It has been also shown that bortezomib, a proteasome inhibitor, exhibited its anti-tumour efficacy by increasing HSP60 and HSP90 expression on the surface of cancer cells and inducing phagocytosis in experimental model of ovarian cancer (Chang et al., Reference Chang, Hsu, Wu, Yang, Wang, Wu and Hung2012).
HSP90
HSP90 is a molecular chaperone important to the stability, folding and activity of over 200 proteins responsible for tumour initiation, development and metastasis. This protein is important for survival and proliferation of protozoan parasites during their intracellular mammalian stage. Since the ATPase activity executed in the N-terminal domain of HSP90 is critical for chaperone functions, HSP90 inhibitors capable to prevent ATP hydrolysis are expected to inhibit HSP90, leading to protein degradation and cell death, making this chaperone an attractive putative therapeutic target for cancer and leishmaniasis treatment (R Woodford et al., Reference R Woodford, Dunn, Ciciarelli, Beebe, Neckers and Mollapour2016; Palma et al., Reference Palma, Ferreira, Petersen, Dias, de Menezes, de Magalhães Moreira, Hernandes and Veras2019; Batista et al., Reference Batista, Ramos, Tassone, Leitão, Montanari, Botta, Mori and Borges2020). 17-N-Allylamino-17-demethoxygeldanamycin (17-AAG, tanespimycin) is an inhibitor of HSP90, which has been investigated in the treatment of cancer such as solid tumours and leukaemia. Alternatively, geldanamycin, and its analogues, 17-dimethylamino ethylamino-17-demethoxygeldanamycin (17-DMAG) and 17-AAG, may show a promising therapeutic activities against leishmaniasis (binding to the N-terminal domain of leishmanial-HSP90). However, the delivery of 17-AAG is difficult because of its poor aqueous solubility (Palma et al., Reference Palma, Ferreira, Petersen, Dias, de Menezes, de Magalhães Moreira, Hernandes and Veras2019; Pires et al., Reference Pires, Magalhaes, Ferrante, de Souza Rebouças, Nguewa, Severino, Barral, Veras and Formiga2020). Moreover, several molecules including Glb02, Glb08, Glb11, Glb16, Glb25 and Glb27 have been suggested as leishmanial-HSP90 inhibitors via binding to the N-terminal region of this protein (Batista et al., Reference Batista, Ramos, Tassone, Leitão, Montanari, Botta, Mori and Borges2020).
Aldehyde dehydrogenase (ALDH)
ALDHs are a group of enzymes found in all subcellular compartments that transform aldehydes to carboxylic acids. In Leishmania, ALDH is located in the mitochondrion (mALDH) and is overexpressed in the promastigote forms compared to the amastigotes (Saxena et al., Reference Saxena, Lahav, Holland, Aggarwal, Anupama, Huang, Volpin, Myler and Zilberstein2007). It has been suggested as a protective protein against oxidative stress during glucose limitation in these parasites (Feng et al., Reference Feng, Feistel, Buffalo, McCormack, Kruvand, Rodriguez-Contreras, Akopyants, Umasankar, David and Jardim2011). Its expression significantly decreases in long culture of Leishmania and mALDH might be related to virulence (Magalhaes et al., Reference Magalhaes, Duarte, Mattos, Martins, Lage, Chavez-Fumagalli, Lage, Menezes-Souza, Regis and Alves2014). Although different ALDH-isoenzymes have been identified in NSCLCs, the expression of such isoenzymes is unknown in Leishmania (Kang et al., Reference Kang, Lee, Hong, Lee, Ahn, Ahn, Seong, Lee, Jang and Hong2016). The low expression of ALDH in normal IMR-90 human lung cells and in Leishmania spp. with attenuated infectivity may highlight the major role of ALDH in the pathogenicity of cancer and leishmaniasis (Bringaud et al., Reference Bringaud, Peris, Zen and Simpson1995; Chavali et al., Reference Chavali, Whittemore, Eddy, Williams and Papin2008; Brocker et al., Reference Brocker, Lassen, Estey, Pappa, Cantore, Orlova, Chavakis, Kavanagh, Oppermann and Vasiliou2010; Feng et al., Reference Feng, Feistel, Buffalo, McCormack, Kruvand, Rodriguez-Contreras, Akopyants, Umasankar, David and Jardim2011; Kang et al., Reference Kang, Lee, Hong, Lee, Ahn, Ahn, Seong, Lee, Jang and Hong2016). The high level of ALDH has been reported in drug-resistant cancer stem cells (Januchowski et al., Reference Januchowski, Wojtowicz and Zabel2013; Clark and Palle, Reference Clark and Palle2016; Vassalli, Reference Vassalli2019). It has also been shown that the use of N,N-diethylaminobenzaldehyde (DEAB) and a combination of gossypol (a pan-ALDH inhibitor) and phenformin leads to cancer cell death (Kang et al., Reference Kang, Lee, Hong, Lee, Ahn, Ahn, Seong, Lee, Jang and Hong2016; Jiménez et al., Reference Jiménez, Pequerul, Amor, Lorenzo, Metwally, Avilés, Parés and Farrés2019). The antiparasitic efficacy of gossypol, as an ALDH inhibitor, has been previously highlighted (Koppaka et al., Reference Koppaka, Thompson, Chen, Ellermann, Nicolaou, Juvonen, Petersen, Deitrich, Hurley and Vasiliou2012). A recent study has identified potential anticancer agents, as potent multi-ALDH isoform inhibitors, increased lipid peroxidation, ROS activity and toxic aldehyde accumulation, and also causing increased apoptosis and G2/M phase cell cycle arrest (Dinavahi et al., Reference Dinavahi, Gowda, Bazewicz, Battu, Lin, Chitren, Pandey, Amin, Robertson and Gowda2020). Such inferred data from cancer cells might clarify the possible function of ALDH in drug-resistant Leishmania strains and suggest the use of ALDH-isoform inhibitors not only for cancer treatment (Dinavahi et al., Reference Dinavahi, Gowda, Bazewicz, Battu, Lin, Chitren, Pandey, Amin, Robertson and Gowda2020), but also as a promising strategy for therapeutic assessments against leishmaniasis.
Topoisomerases (TOPs)
TOPs are a group of enzymes that catalyse changes in the DNA topology during replication, transcription, recombination and genome repair. Firstly, they repeatedly can cut and join phosphodiester bonds from the phosphate deoxyribose structure which harbouring nitrogenous bases encoding genetic message. Secondly, they allow other DNA chains to pass between the temporary formed tails, by using energy from the nucleotide linkage and bind covalently to 3′ or 5′-DNA end (Wang, Reference Wang2002; Pommier et al., Reference Pommier, Sun, Shar-yin and Nitiss2016).
TOP IB: Type IB TOPs cut one DNA strand and covalently join to 3′ end. Two types of TOP IB inhibitors have been described: type I inhibitors or poisons, which stabilize the cleavage complex by creating a ternary complex DNA–enzyme drug; and type II inhibitors which act on the catalytic function of the enzyme. Despite their relevant role in genetic information fidelity conservation, TOP IB from Leishmania parasites are heterodimer enzymes which deeply differ from those of humans. For this reason, Leishmania TOP IB are considered as potential therapeutic targets. Camptothecins and their derivatives have demonstrated inhibitory activities against these parasitic enzymes (Prada et al., Reference Prada, Alvarez-Velilla, Balana-Fouce, Prieto, Calvo-Alvarez, Escudero-Martínez, Requena, Ordóñez, Desideri and Perez-Pertejo2013). Synthetic indenoisoquinolines are potent TOP IB inhibitors with leishmanicidal activity ‘in vivo’ (Balaña-Fouce et al., Reference Balaña-Fouce, Prada, Requena, Cushman, Pommier, Álvarez-Velilla, Escudero-Martínez, Calvo-Álvarez, Pérez-Pertejo and Reguera2012). Recently, hybrids of isoquinolines and camptothecins have shown their leishmanicidal activity through TOP IB activity inhibition (Reguera et al., Reference Reguera, Álvarez-Velilla, Domínguez-Asenjo, Gutiérrez-Corbo, Balaña-Fouce, Cushman and Pérez-Pertejo2019). Similarly, natural alkaloids and marine fatty acids have been reported as Leishmania TOP IB inhibitors (Carballeira et al., Reference Carballeira, Cartagena, Prada, Rubio and Balaña-Fouce2009, Reference Carballeira, Montano, Cintrón, Márquez, Rubio, Prada and Balaña-Fouce2011, Reference Carballeira, Cartagena, Li, Chen, Prada, Calvo-Alvarez, Reguera and Balaña-Fouce2012a, Reference Carballeira, Cartagena, Sanabria, Tasdemir, Prada, Reguera and Balaña-Fouce2012b, Reference Carballeira, Montano, Alvarez-Velilla, Prada, Reguera and Balaña-Fouce2013; Chowdhury et al., Reference Chowdhury, Mukherjee, Sengupta, Chowdhury, Mukhopadhyay and Majumder2011; Kumar et al., Reference Kumar, Chowdhury, Jatte, Chakrabarti, Majumder, Jha and Mukhopadhyay2015, Reference Kumar, Chowdhury, Sarkar, Chakrabarti, Majumder, Jha and Mukhopadhyay2016; Pérez-Pertejo et al., Reference Pérez-Pertejo, Escudero-Martínez, Reguera, Balaña-Fouce, García, Jambrina, San Feliciano and Castro2019) as well as anticancer compounds (Carballeira et al., Reference Carballeira, Montano, Amador, Rodríguez, Golovko, Golovko, Reguera, Álvarez-Velilla and Balaña-Fouce2016, Reference Carballeira, Morales-Guzman, Alvarez-Benedicto, Torres-Martinez, Delgado, Griebenow, Tinoco, Reguera, Perez-Pertejo and Carbajo-Andres2018).
On the other hand, human TOP I is a monomeric enzyme which has been demonstrated being overexpressed in several cancers (Lynch et al., Reference Lynch, Bronstein and Holden2001; Berney et al., Reference Berney, Shamash, Gaffney, Jordan and Oliver2002; Gouveris et al., Reference Gouveris, Lazaris, Papathomas, Nonni, Kyriakou, Delladetsima, Patsouris and Tsavaris2007; Kümler et al., Reference Kümler, Balslev, Stenvang, Brunner and Nielsen2015). Most of the marketed TOP inhibitors applied for cancer treatment target TOP type II. However, there are camptothecin derivatives approved such as irinotecan, topotecan and belotecan (Hevener et al., Reference Hevener, Verstak, Lutat, Riggsbee and Mooney2018) which use TOP IB as a target.
TOP II: Type II TOPs are homodimeric enzymes responsible for catalysing topological changes in the DNA by transitory break of both nucleotide chains. During this process an intermediate covalent, between 5′-ends and such enzymatic subunits, is formed. Those proteins are conserved in blood parasites such as Plasmodium, Trypanosoma or Leishmania and their mammal hosts. However, the emerging interest on Leishmania TOP II was mainly due to its involvement in the kinetoplast DNA network and its replication. Moreover, TOP II is related to drug resistance in these parasites (Jayanarayan and Dey, Reference Jayanarayan and Dey2003; Sengupta et al., Reference Sengupta, Mukherjee, Das, Mandal, Das, Mukherjee and Majumder2005; Singh et al., Reference Singh, Thakur, Chakraborti and Dey2009). The overexpression of a TOP II-like enzyme activity has highlighted the regulatory function of this putative enzyme in arsenite-resistant L. donovani strains (Jayanarayan and Dey, Reference Jayanarayan and Dey2003). A point mutation, R250G, has been detected in the ATPase domain of the TOP II in arsenite-resistant strain of L. donovani parasite. The variation in the TOP II gene sequence between arsenite-sensitive and -resistant strains is anticipated to be responsible for the varied behaviour of this enzymes in response to antileishmanial/anti-TOP II agents (Singh et al., Reference Singh, Thakur, Chakraborti and Dey2009). As aforementioned, similarly to TOP IB targeting agents, there are two groups of TOP II inhibitors depending of their mode of action, and some of them are even able to target both, type I and type II TOPs (Ray et al., Reference Ray, Sadhukhan, Mandal, Mahato and Majumder1997). For instance, isobenzofuranone derivatives are capable to inhibit Leishmania TOP II linked to DNA (Mishra et al., Reference Mishra, Vinayagam, Saha, Chowdhury, Roychowdhury, Jaisankar and Majumder2014; Chowdhury et al., Reference Chowdhury, Godinho, Vinayagam, Zuma, Silva, Jaisankar, Rodrigues, De Souza and Majumder2018), whereas protoberberine perform its effect by stabilizing TOP II–DNA cleavage complex (Marquis et al., Reference Marquis, Makhey, LaVoie and Olivier2003). Mitonafide had demonstrated its activity on Leishmania nuclear and kinetoplast-TOP II (Slunt et al., Reference Slunt, Grace, Macdonald and Pearson1996). Recently, a mitonafide derivative has shown a Leishmania TOP II inhibitory effect similar to type I inhibitors and at the mRNA level (Fernández-Rubio et al., Reference Fernández-Rubio, Larrea, Guerrero, Herrero, Gamboa, Berrio, Plano, Amin, Sharma and Nguewa2019).
Human TOP II presents two isoforms: alpha (TOP 2A) and beta (TOP 2B) which exhibit differences in their molecular weight, genetic regulation and the location of the active site. TOP II expression in cancer lines has been largely studied (Doyle, Reference Doyle1994). Although TOP 2B isoform is expressed relatively constant throughout the cell cycle in both, normal and transformed cells, TOP 2A has been found abnormally expressed in different cancers such as breast, lung or prostate among others (Giaccone et al., Reference Giaccone, van Ark-Otte, Scagliotti, Capranico, van der Valk, Rubio, Dalesio, Lopez, Zunino and Walboomers1995; Depowski et al., Reference Depowski, Rosenthal, Brien, Stylos, Johnson and Ross2000; Mrklic et al., Reference Mrklic, Pogorelic, Capkun and Tomic2014; Schaefer-Klein et al., Reference Schaefer-Klein, Murphy, Johnson, Vasmatzis and Kovtun2015; An et al., Reference An, Xu, Luo, Zheng, Lu, Yang, Qin, Yuan, Shi and Jiang2018; Liu et al., Reference Liu, Xiong, Lin, Yang, Dang and Chen2018). In fact, there are marketed anticancer drugs targeting TOP II, such as anthracycline and podophyllotoxin derivatives (Hevener et al., Reference Hevener, Verstak, Lutat, Riggsbee and Mooney2018). Etoposide, which belongs to the last group, is the best known TOP II poison, stabilizing DNA cleavage complex. Currently, TOP II molecules continue being a promising therapeutic target against cancer, and numerous research projects have focused on the synthesis of new inhibitors (Liberio et al., Reference Liberio, Sadowski, Davis, Rockstroh, Vasireddy, Lehman and Nelson2015; Karelia et al., Reference Karelia, Sk, Singh, Gowda, Pandey, Ramisetti, Amin and Sharma2017; Jiang et al., Reference Jiang, Zhang, Li, Wang, Dong, Zhang, Chen and Du2018; Yamashita et al., Reference Yamashita, Tahara, Hayakawa, Matsumoto, Wada, Tomioka and Iida2018; Li et al., Reference Li, Jiang, Zhang, Li, Zhao, Zhou, Zhang, Tang, Dong and Huang2018b).
Proliferating cell nuclear antigen
PCNA is a processivity factor for DNA polymerase delta (Pol δ) and epsilon (Pol ɛ). It also interacts with other proteins involved in cell-cycle progression which are not parts of the DNA polymerase complex. PCNA has demonstrated its role in the replication and repair of DNA, chromatin assembly and RNA transcription. Its importance in Leishmania pathology is related to its significant role in drug response in clinical isolates (Tandon et al., Reference Tandon, Chandra, Baharia, Das, Misra, Kumar, Siddiqi, Sundar and Dube2014). In addition, this protein is a potential therapeutic target against leishmaniasis since it showed susceptibility to be inhibited at the mRNA level by selenocompounds (Fernández-Rubio et al., Reference Fernández-Rubio, Campbell, Vacas, Ibañez, Moreno, Espuelas, Calvo, Palop, Plano and Sanmartin2015; Fernández-Rubio et al., Reference Fernández-Rubio, Larrea, Guerrero, Herrero, Gamboa, Berrio, Plano, Amin, Sharma and Nguewa2019). It is known that PCNA is overexpressed in tumour cells, to adapt the high capacity of such cells to exhibit an uncontrolled replication (Naryzhny and Lee, Reference Naryzhny and Lee2007). Interestingly, there are several small molecules, including cell-penetrating peptides, targeting PCNA with promising results against breast cancer and other tumours. For instance, several compounds inhibit the association of PCNA and chromatin, resulting in apoptosis and DNA damage in prostate and lung cancer (Dillehay et al., Reference Dillehay, Lu and Dong2014; Lu and Dong, Reference Lu and Dong2019). A cell penetrating peptide had been described as caspase-dependent apoptosis inductor which increased the activity of antitumour treatments in multiple myeloma cells (Muller et al., Reference Muller, Misund, Holien, Bachke, Gilljam, Våtsveen, Rø, Bellacchio, Sundan and Otterlei2013).
Tubulins
Tubulins are highly conserved dimeric proteins present in all eukaryotes. Alpha-beta (α/β) dimers polymerize to form microtubules, which serve as a skeletal system for living cells and participate in several essential functions, such as mitosis or intracellular transport among others (Montecinos-Franjola et al., Reference Montecinos-Franjola, Chaturvedi, Schuck and Sackett2019). In Leishmania, α-tubulin is a key component of the cytoskeleton, responsible for cell shape and involved in cell division, ciliary and flagellar motility (Ramírez et al., Reference Ramírez, Requena and Puerta2013). In addition, it has been related to drug resistance (Prasad et al., Reference Prasad, Kumar and Dey2000; Jayanarayan and Dey, Reference Jayanarayan and Dey2004). Furthermore, proteomic analyses demonstrated that this protein is more abundant in Sb(III)-resistant Leishmania cell lines (Matrangolo et al., Reference Matrangolo, Liarte, Andrade, de Melo, Andrade, Ferreira, Santiago, Pirovani, Silva-Pereira and Murta2013). Due to its role in Leishmania biology and pathology, α-tubulin has been considered a promising target against leishmaniasis and, consequently, compounds targeting this protein have been tested. Selenocompounds were able to significantly reduced α-tubulin gene expression (Fernández-Rubio et al., Reference Fernández-Rubio, Campbell, Vacas, Ibañez, Moreno, Espuelas, Calvo, Palop, Plano and Sanmartin2015). Podophyllum derived toxins are Leishmania tubulin inhibitors however, those showed discrepancies between protein activity and parasite growth inhibition (Escudero-Martínez et al., Reference Escudero-Martínez, Pérez-Pertejo, Reguera, Castro, Rojo, Santiago, Abad, García, López-Pérez and San Feliciano2017). Such results differ from those of colchicine against trypanosomatids. This potent inhibitor of tubulin polymerization in higher eukaryotes seems to lack activity against these parasites, probably due to conformational changes in the protein which block colchicine access (Luis et al., Reference Luis, Serrano, Hidalgo and Mendoza-León2013).
Alterations in the expression of tubulin are related to drug resistance in different cancers, including breast, lung, ovarian, gastric and prostate (Bernard-Marty et al., Reference Bernard-Marty, Treilleux, Dumontet, Cardoso, Fellous, Gancberg, Bissery, Paesmans, Larsimont and Piccart2002; Hwang et al., Reference Hwang, Hong, Kim, Kim, Choi, Kim, Jung, Shim, Bae and Hwang2013; Jiang et al., Reference Jiang, Yu, Zhou, Wang and Su2013; Tsourlakis et al., Reference Tsourlakis, Weigand, Grupp, Kluth, Steurer, Schlomm, Graefen, Huland, Salomon and Steuber2014; Du et al., Reference Du, Li, Fang, Liu, Wang, Li, Zhou and Wang2015). Depending of their mechanism of action, tubulin inhibitors could be mainly grouped as microtubule-destabilizing agents or microtubule-stabilizing agents (Perez, Reference Perez2009). The firsts are colchicine analogues and vinca alkaloids. The seconds are paclitaxel derivatives. Colchicine analogues bind to the colchicine binding site (CBS), one of the most important pockets for potential tubulin polymerization destabilizers. These compounds inhibit tubulin assembly and suppress microtubule formation (Lu et al., Reference Lu, Chen, Xiao, Li and Miller2012). Nevertheless, currently there are not FDA (Food and Drug Administration) approved tubulin inhibitors targeting the CBS (Li et al., Reference Li, Jiang, Li, Liu, Su and Chen2018a, Reference Li, Jiang, Zhang, Li, Zhao, Zhou, Zhang, Tang, Dong and Huang2018b). Taxanes, such as paclitaxel and its derivatives, bind to the interior surface of microtubules, resulting in their stabilization. Therefore, microtubules stabilization increase, leading to cell cycle arrest and apoptosis (Jordan, Reference Jordan2002; Jordan and Wilson, Reference Jordan and Wilson2004).
Voltage-dependent anion-selective channel protein 1 (VDAC-1)
VDAC-1 forms a large channel in the outer mitochondrial membrane that allows the diffusion of hydrophilic molecules. Apoptosis, metabolic flux and intracellular signalling are also important functions of this porin in eukaryotes. Leishmania parasites use anionic voltage-dependent channels as a transport system for adaption to nutritional stress conditions and pH homoeostasis (Vieira et al., Reference Vieira, Lavan, Dagger and Cabantchik1994; Lawen et al., Reference Lawen, Ly, Lane, Zarschler, Messina and De Pinto2005; Shoshan-Barmatz et al., Reference Shoshan-Barmatz, Israelson, Brdiczka and Sheu2006; Bayrhuber et al., Reference Bayrhuber, Meins, Habeck, Becker, Giller, Villinger, Vonrhein, Griesinger, Zweckstetter and Zeth2008).
In cancer cells, VDAC-1 is a protein with dual function involved in the regulation of survival and mitochondria-mediated apoptosis (Shoshan-Barmatz et al., Reference Shoshan-Barmatz, Krelin, Shteinfer-Kuzmine and Arif2017). It has been shown that the use of VDAC-1-specific small interfering RNA leads to the metabolism alteration and the growth suppression of cancer cells. Moreover, the up-regulation of the VDAC-1 increases the expression of apoptotic proteins such as hexokinase (HK), B-cell lymphoma-xL (Bcl-xL) and Bcl-2 in cancer cells and leads to the growth inhibition of such cells. Ion channel blockers have demonstrated activity against ion channel proteins in both, cancer cells and Leishmania parasites (Ponte-Sucre et al., Reference Ponte-Sucre, Campos, Fernandez, Moll and Mendoza-León1998; Kale et al., Reference Kale, Amin and Pandey2015; Leanza et al., Reference Leanza, Manago, Zoratti, Gulbins and Szabo2016; Reimão et al., Reference Reimão, Mesquita, Ferreira and Tempone2016; Shoshan-Barmatz et al., Reference Shoshan-Barmatz, Krelin, Shteinfer-Kuzmine and Arif2017). Verapamil and nifedipine have been identified as human calcium channel blockers in cancer therapy which had been proposed to have mild anti-leishmanial activity (Kashif et al., Reference Kashif, Manna P, Akhter, Alaidarous and Rub2017). Leishmania donovani Ca2+ ion channel (Ld-CC) has been suggested as potential drug target in leishmaniasis treatment (Kashif et al., Reference Kashif, Manna P, Akhter, Alaidarous and Rub2017). Ld-CC regulates Ca2+ concentration which is involved in several functions such as mitochondrial oxidative metabolism and entry inside the macrophages and flagellar motion. Two ligands, ZINC17287336 and ZINC29590262 were showed highest binding affinity towards Ld-CC. These selected compounds have relatively more binding affinity than verapamil and nifedipine. Since ZINC29590262 has shown poor binding affinity towards the human voltage-dependent L-type calcium channel subunit alpha-1C in comparison with the Ld-CC, this compound can be suggested as an appropriate drug target (40% more binding affinity with Ld-CC than the human-voltage-dependent calcium channel) (Kashif et al., Reference Kashif, Manna P, Akhter, Alaidarous and Rub2017).
Mitochondrial import receptor subunit (TOM-40)
TOM-40 is located at the core of the translocase of the outer membrane (TOM) structure. Data produced by genome sequencing in protozoa has indicated the presence of TOM-40 homologues in Cryptosporidium (Keithly et al., Reference Keithly, Langreth, Buttle and Mannella2005; Umejiego et al., Reference Umejiego, Gollapalli, Sharling, Volftsun, Lu, Benjamin, Stroupe, Riera, Striepen and Hedstrom2008). Recent studies have reported TOM-40 in L. infantum amastigotes, and a protein with low similarity to TOM-40 in Trypanosoma (Zarsky et al., Reference Zarsky, Tachezy and Dolezal2012; Rashidi et al., Reference Rashidi, Mojtahedi, Shahriari, Kalantar, Ghalamfarsa, Mohebali and Hatam2019). Although, the function of TOM-40 in Leishmania is unknown, bioinformatics data of the T. brucei genome for both TOM-40 and VDAC have identified a single open reading frame, with sequence analysis suggesting that TOM-40s and VDACs are ancestrally related and should be classified into the same protein family (the mitochondrial porins) (Pusnik et al., Reference Pusnik, Charrière, Maser, Waller, Dagley, Lithgow and Schneider2009). This information might open an attractive insight about using ion channel blockers against both TOM-40s and VDACs in Kinetoplastida such as Trypanosoma and Leishmania parasites.
As a tumour marker, TOM-40 is up-regulated in ovarian cancer cells and induces the proliferation and metastasis of these cells ‘in vitro’. It seems that TOM-40 increases the replication of cancer cells through regulating the mitochondrial activity and improving cellular energy and redox status (Yang et al., Reference Yang, Shin, Cho, Chung, Lee, Kim and Kang2020). Evidence has shown that the inhibition of TOM-40 expression in ovarian cancer cells leads to a reduction in the proliferation and migration of cancer cells. However, directly targeting TOM-40 may be challenging in clinical application due to its substantial expression in normal cells. Since metformin (first-line therapy for type 2 diabetes) has been already clinically used with lower side-effects, this compound can be an appropriate alternative drug for targeting TOM-40 and the mitochondria (inhibiting mitochondria complex I) in epithelial ovarian cancer (Yang et al., Reference Yang, Shin, Cho, Chung, Lee, Kim and Kang2020). The pathogenic functions of TOM-40 and therapeutic strategy against this target in cancer treatment might persuade the scientists to further investigate the expression and possible functions of TOM-40 in pathogenicity of leishmaniasis.
Ornithine aminotransferase (OAT)
Aminotransferase are important enzymes that are able to transaminase aromatic amino acids. The functions of OAT are related to l-arginine pathways involved in polyamines production (Muxel et al., Reference Muxel, Aoki, Fernandes, Laranjeira-Silva, Zampieri, Acuña, Müller, Vanderlinde and Floeter-Winter2018). Polyamines metabolism is strongly important for Leishmania cell proliferation and infection (Ilari et al., Reference Ilari, Fiorillo, Baiocco, Poser, Angiulli and Colotti2015). For instance, a recent study has evaluated the effect of polyamine depletion in L. donovani mutants lacking ornithine decarboxylase or spermidine synthase. As mentioned in Table 1, DFMO inhibitor of ornithine decarboxylase, the enzyme that catalyses putrescine biosynthesis. Those results suggested that putrescine is not only a precursor metabolite for spermidine formation; it had specific functions for parasite viability and proliferation. These results also elucidated that ornithine decarboxylase inhibition and putrescine depletion was the most promising strategy for targeting polyamine biosynthetic pathway. It seemed that both polyamines (ornithine decarboxylase or spermidine) were required for parasite survival but that the presence of either putrescine or spermidine alone may allow Leishmania parasites to survive in a quiescent-like state for several weeks (Perdeh et al., Reference Perdeh, Berioso, Love, LoGiudice, Le, Harrelson and Roberts2020).
OAT as a β-catenin target gene in the liver is involved in the metabolism of glutamine. It has been shown that in hepatocellular carcinoma (HCC), the expression of OAT is up-regulated and the mechanism of this gene up-regulation is related to the activation of β-catenin signalling (Cadoret et al., Reference Cadoret, Ovejero, Terris, Souil, Levy, Lamers, Kitajewski, Kahn and Perret2002; Colnot et al., Reference Colnot, Decaens, Niwa-Kawakita, Godard, Hamard, Kahn, Giovannini and Perret2004). This information proposed that OAT, β-catenin signalling and the metabolism of glutamine are important factors in carcinogenesis especially in HCC (Cadoret et al., Reference Cadoret, Ovejero, Terris, Souil, Levy, Lamers, Kitajewski, Kahn and Perret2002; Thompson and Monga, Reference Thompson and Monga2007). The existence of inhibitors targeting OAT used to block the proliferation of HCC, may also allow the selection of this transferase as a therapeutic target against Leishmania parasites (Zigmond et al., Reference Zigmond, Ya'acov, Lee, Lichtenstein, Shalev, Smith, Zolotarov, Ziv, Kalman and Le2015).
Selenoproteins and selenoamino acid
Selenoproteins are a group of enzymes bearing selenocysteine in their catalytic domain. Many of the Se-bearing proteins participate in oxidative stress protection as observed in Leishmania parasites (Iribar et al., Reference Iribar, Tosi and Cruz2003; Da Silva et al., Reference Da Silva, Silva-Jardim and Thiemann2014). The use of leishmanial selenoproteins and selenoamino acid (selenomethionine) as therapeutic targets due to their role in modulation and evasion of the host immune responses has been recently suggested (Rashidi et al., Reference Rashidi, Nguewa, Mojtahedi, Shahriari, Kalantar and Hatam2020a, Reference Rashidi, Kalantar, Nguewa and Hatam2020b).
The role of selenoproteins and their metabolites including methylselenol, selenodiglutathione, Se-methylselenocysteine and selenomethionine has been underlined in the metabolism of lung cancer cells (Seng and Tiekink, Reference Seng and Tiekink2012). Such metabolites inhibit protein kinases and alter cell cycle in cancer cells. Furthermore, these metabolites induce the activity of lymphokine-activated killer cells and natural killer cells and finally stimulate the immune system against cancer cells (Seng and Tiekink, Reference Seng and Tiekink2012). The use of leishmanial selenoproteins inhibitors as well as the anti-tumour property of selenoproteins and their metabolites against cancer cells might suggest that such aforementioned compounds represent therapeutic targets for the treatment of leishmaniasis and cancers. Auranofin is gold-containing compound with well-known selenoproteins synthesis inhibitor properties. Its activity has been demonstrated against thioredoxin reductase (TrxRd), a Se-bearing enzyme involved in maintaining the intracellular redox state. In cancer, TrxRd overexpression is related to the aggressiveness of the malignancy (Kahlos et al., Reference Kahlos, Soini, Saily, Koistinen, Kakko, Paakko, Holmgren and Kinnula2001; Lincoln et al., Reference Lincoln, Ali, Tonissen and Clarke2003) and its inhibition lead to apoptosis of tumour cells (You and Park, Reference You and Park2016). Using drug repurposing strategy, auranofin had been tested against parasitic diseases including Leishmania, with promising results. However, this compound seems not to target selenoproteins in these parasites, but rather interact with trypanothione reductase, a key enzyme of Leishmania polyamine-dependent redox metabolism (Ilari et al., Reference Ilari, Baiocco, Messori, Fiorillo, Boffi, Gramiccia, Di Muccio and Colotti2012; Sharlow et al., Reference Sharlow, Leimgruber, Murray, Lira, Sciotti, Hickman, Hudson, Leed, Caridha and Barrios2014; Manhas et al., Reference Manhas, Gowri and Madhubala2016). Nevertheless, the existence of specific selenoproteins inhibitors with effective activities against cancer cells might support the role of leishmanial selenoproteins as therapeutic targets (Yan et al., Reference Yan, Guo, Wang, Mao, Huang and Li2015; Arnér, Reference Arnér2017).
Phosphoglycerate kinase-1 [PGK-1 (PKG-B)]
PGKs are transferases involved in ATP production from ADP and 1,3-diphosphoglycerate. Their involvement in the glycolytic pathway and survival of Leishmania parasites has been previously reported (Hart and Opperdoes, Reference Hart and Opperdoes1984; Blattner et al., Reference Blattner, Swinkels, Dörsam, Prospero, Subramani and Clayton1992; Azevedo et al., Reference Azevedo, Toledo, Defina, Pedrosa and Cruz2015). PGKs (PGK-B) are overexpressed in antimony-resistant strains of Leishmania. Therefore, they might be related to the pathogenicity of these parasites (Blattner et al., Reference Blattner, Helfert, Michels and Clayton1998; Kazemi-Rad et al., Reference Kazemi-Rad, Mohebali, Khadem-Erfan, Saffari, Raoofian, Hajjaran, Hadighi, Khamesipour, Rezaie and Abedkhojasteh2013). Increased level of glycolysis enzymes such as PGK in the antimony resistant Leishmania isolates suggesting resistant strains require higher energy to protect against antimony-induced oxidative stress. Alternatively, the overexpression of such enzyme might lead to enhancing in pyruvate which can remove peroxides and participate to reduce oxidative stress (Biyani et al., Reference Biyani, Singh, Mandal, Chawla and Madhubala2011). In both, Leishmania and mammalian cells, PGKs are encoded by two genes: gene B and gene C, and PGK-1 and PGK-2, respectively (Watson and Littlechild, Reference Watson and Littlechild1990; McKoy et al., Reference McKoy, Badal, Prescott, Lux and Hart1997). Several monosubstituted N6 and N2 adenosine derivatives were selected to screen against T. brucei PGK. Of these, 2-amino-N6-substituted analogues represented appropriate activity against the parasite kinase compared with the N6 compounds that lacked the C2 amino group, although activity was still weak (Merritt et al., Reference Merritt, Silva, Tanner, Stuart and Pollastri2014). Since protein kinase inhibition has been primarily discussed as anti-trypanosomatid strategy in treatment, these proteins such as PGK can further investigated in leishmaniasis.
The importance of PGK-1 in cancer development resides on its involvement in drug-resistance and its dual action depending on the cellular environment. Under intracellular hypoxia conditions, it plays an oncogenic role. However, it decreases tumour growth when it is secreted extracellularly through angiogenesis inhibition (Daly et al., Reference Daly, Wind, Jiang, Sun and Hogg2004; He et al., Reference He, Luo, Zhang, Wang, Zhang, Li, Ejaz and Liang2019). In addition to the metabolic functions of PGK-1 in cancer cells, this enzyme induces and increases the angiostatin formation and leads to the restriction of angiogenesis in tumours. The anti-tumour property of PGK-1 has been shown in Lewis lung carcinoma (LLC-1). It is well known that cyclooxygenase-2 (COX-2), as an important marker of resistance to apoptosis in cancer cells, promotes angiogenesis and metastasis (Tsujii and DuBois, Reference Tsujii and DuBois1995; Tsujii et al., Reference Tsujii, Kawano, Tsuji, Sawaoka, Hori and DuBois1998). Due to the overexpression of PGK-1 in LLC-1, COX-2 is decreased and therefore, cell invasion, prostaglandin E2 and angiogenesis are affected by this mechanism. Finally, the progression of cancer cells is then restricted (Tang et al., Reference Tang, Ho, Cho, Lin, Sun, Chi, Wang, Jhou, Yang and Sun2008; Ho et al., Reference Ho, Tang, Ng, Yang, Leu, Lin, Feng, Sung and Sun2010). Under solid tumours and hypoxia conditions, PGK-1 is the main source of production of ATP. Solid tumour cells use mechanisms that inhibit the production of PGK and decrease the angiostatin formation. Furthermore, other angiogenesis activators such as vascular endothelial cell growth factor are active in solid tumours (Daly et al., Reference Daly, Wind, Jiang, Sun and Hogg2004). Currently, potential PGKs-inhibitors are under development (He et al., Reference He, Luo, Zhang, Wang, Zhang, Li, Ejaz and Liang2019).
Conclusion
The up- and down-regulation of the aforementioned proteins were mostly related to the survival, development, pathogenicity, metabolic pathways and vital signalling in Leishmania parasites and cancer cells. As an interesting issue, the regulation of these markers can be investigated in interactions that can be occurred between Leishmania parasites and cancer cells under ‘in vivo’ and ‘in vitro’ conditions. The reliable validation of the expression of such proteins in Leishmania parasites and cancer cells using further experiments is warranted to subsequently confirm their possible functions. Further investigation of the differentially regulation of common expressed proteins between cancer cells, normal human cells, low-pathogenic and high-pathogenic forms of Leishmania parasites might elucidate novel and attractive information concerning such proteins. The existence of common triggering factors reflects mutual features in the etiopathogenetic mechanisms underlying leishmaniasis and cancer. Given these similarities, lessons learned from strategies against cancer may be relevant to design adequate approaches to reduce and eliminate leishmaniasis. Herein, we focused specifically on the shared mechanisms at protein scale. Taken together, the introduction of common expressed proteins in Leishmania parasites and cancer cells might reveal valuable information regarding the possible common mechanisms of pathogenicity and therapeutic targets in leishmaniasis and cancers. Taking into account that current therapies for neglected diseases are based in drugs lacking effectiveness, the lack of new specific anti-Leishmania compounds and of research focused on this group of diseases, drug repurposing constitutes a useful tool to find effective candidates in leishmaniasis control and elimination. This review reinforces the likely functional similarities between many proteins in cancer and parasites, some of them being recognized therapeutic targets and thus the potential use of drugs with proven efficacy in the treatment of cancer for treating parasitic diseases and vice versa, opening new avenues to the one health approach.
Acknowledgements
PN gratefully acknowledges support provided by Fundación La Caixa (LCF/PR/PR13/11080005) and Fundación Caja Navarra, Gobierno Navarra Salud (12/2017), Fundación Roviralta, Ubesol, Government of Navarre, Laser Ebro and Inversores Garcilaso de la Vega S.L.
Financial support
This study was supported by the National Institute for Medical Research Development (NIMAD), Tehran, Iran, grant number 971405.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable.






