Introduction
American tegumentary leishmaniasis (ATL) includes cutaneous leishmaniasis (CL), mucocutaneous (MCL) and mucosal leishmaniasis (ML), and affects 1–2 million people in the Americas (Reithinger et al., Reference Reithinger, Dujardin, Louzir, Pirmez, Alexander and Brooker2007). Localized CL (LCL) is generally a self-healing disease characterized by ulcerative, nodular or verrucous lesions on the skin caused by members of the Leishmania complex and endemic to many parts of the world including Brazil and Peru (Reithinger et al., Reference Reithinger, Dujardin, Louzir, Pirmez, Alexander and Brooker2007; Aronson et al., Reference Aronson, Herwaldt, Libman, Pearson, Lopez-Velez, Weina, Carvalho, Ephros, Jeronimo and Magill2016). Other clinical manifestations of CL include inflammatory CL where ulcers are associated with erythema, purulent exudate, pain and/or lymphatic involvement and more recently, atypical cutaneous leishmaniasis, which has been documented in an endemic region of Brazil (Guimares et al., Reference Guimares, Queiroz, Silva, Silva, Magalhaes, Lago, Machado, Bacellar, Wilson, Beverley, Carvalho and Schriefer2016). To add, other forms include diffuse cutaneous leishmaniasis with multiple non-ulcerative nodules (Reithinger et al., Reference Reithinger, Dujardin, Louzir, Pirmez, Alexander and Brooker2007), and disseminated leishmaniasis, defined as maculopapular lesions identified in 2 or more anatomical sites ranging from 10 to 300 in number (Guimares et al., Reference Guimares, Queiroz, Silva, Silva, Magalhaes, Lago, Machado, Bacellar, Wilson, Beverley, Carvalho and Schriefer2016). ML is a form of the disease affecting mucous membranes such as the nose, mouth, pharynx and larynx, more often attributed to sequela of the initial CL infection in Latin America, while MCL involves both cutaneous and mucosal lesions (Reithinger et al., Reference Reithinger, Dujardin, Louzir, Pirmez, Alexander and Brooker2007). This diverse phenotypology reflects a complex relationship between host, parasite and vector factors (extensively reviewed in Reithinger et al., Reference Reithinger, Dujardin, Louzir, Pirmez, Alexander and Brooker2007), with strong geographic- and species-specific preponderances to cutaneous manifestations of disease.
To add to this complexity of ATL pathogenesis, the presence of a double-stranded RNA virus, Leishmania RNA virus-1 (LRV-1), has been identified in up to a quarter of certain strains of Leishmania Viannia spp., including L. V. braziliensis and L. V. guyanensis. LRV-1 found in New World Viannia strains are identified as LRV-1, with 14 subtypes (LRV-1-1 to LRV-1-14) predominantly found in the Amazon basin (Hartlet et al., Reference Hartlet, Ronet, Zangger, Beverley and Fasel2012; Ginouves et al., Reference Ginouves, Simon, Bourreau, Lacoste, Ronet, Couppie, Nacher, Demar and Prevot2016). Genetic diversity between LRV-1 and parasite species exists, however the viruses from the same parasite species have shown less heterogeneity (Catanhede et al., Reference Cantanhede, Fernandes, Ferreira, Porrozzi, Ferreira and Cupolillo2018). In South America, it is believed that 10–15% of LCL will progress to either MCL or ML months to years after healing of the initial LCL lesion (Ives et al., Reference Ives, Ronet, Prevel, Ruzzante, Fuertes-Marraco, Schutz, Zangger, Revaz-Breton, Lye, Hickerson, Beverley, Acha-Orbea, Launois, Fasel and Masina2011; Ronet et al., Reference Ronet, Beverley and Fasel2011; Valencia et al., Reference Valencia, Adaui, Chantry, Alba, Ramos, Arevalo, Llanos-Cuentas and Boggild2014). It is hypothesized that the presence of LRV-1 will advance CL to MCL/ML stemming from an over-active immune response leading to severe immunopathological tissue infiltration and destruction (Ogg et al., Reference Ogg, Carrion, de Carvalho Botelho, Mayrink, Correa-Oliveira and Patterson2003; Ives et al., Reference Ives, Ronet, Prevel, Ruzzante, Fuertes-Marraco, Schutz, Zangger, Revaz-Breton, Lye, Hickerson, Beverley, Acha-Orbea, Launois, Fasel and Masina2011; Ronet et al., Reference Ronet, Beverley and Fasel2011; Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017).
LRV-1 has been documented in 20–25% of clinical isolates of L. V. guyanensis and L. V. braziliensis found in Brazil and Peru and has been associated with first-line treatment failure (Ives et al., Reference Ives, Ronet, Prevel, Ruzzante, Fuertes-Marraco, Schutz, Zangger, Revaz-Breton, Lye, Hickerson, Beverley, Acha-Orbea, Launois, Fasel and Masina2011; Bourreau et al., Reference Bourreau, Ginouve, Prevot, Hartley, Gangneux, Robert-Gangneux, Dufour, Marie, Bertolotti, Pratlong, Martin, Schutz, Couppie, Fasel and Ronet2016). Studies have also indicated higher levels of LRV-1 in metastasizing vs non-metastasizing strains of L. V. guyanensis, which were correlated to increased levels of proinflammatory cytokines and chemokines including tumour necrosis factor (TNF)-α, interleukin (IL)-6, chemokine ligand 10 (CXCL10), chemokine ligand 4 (CCL4) and chemokine ligand 5 (CCL5) after recognition by Toll-like receptor 3 in human and murine studies (Ives et al., Reference Ives, Ronet, Prevel, Ruzzante, Fuertes-Marraco, Schutz, Zangger, Revaz-Breton, Lye, Hickerson, Beverley, Acha-Orbea, Launois, Fasel and Masina2011). On the other hand, in a human macrophage model, LRV-1 in L. V. braziliensis was correlated to lower expression levels of TNF-α, IL-6, IL-1β, CXCL10 and increases in superoxide dismutase, although these differences were not noted in the analysis of 5 L. V. panamensis strains (Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017). Given that LRV-1 may predict and correlate to more severe clinical manifestations of ATL, we aimed to understand its prevalence in clinical isolates of L. V. panamensis, a species in which LRV-1 is not well described, and the possible epidemiologic association between severe and non-severe phenotypes of ATL.
Materials and methods
Specimen enrolment
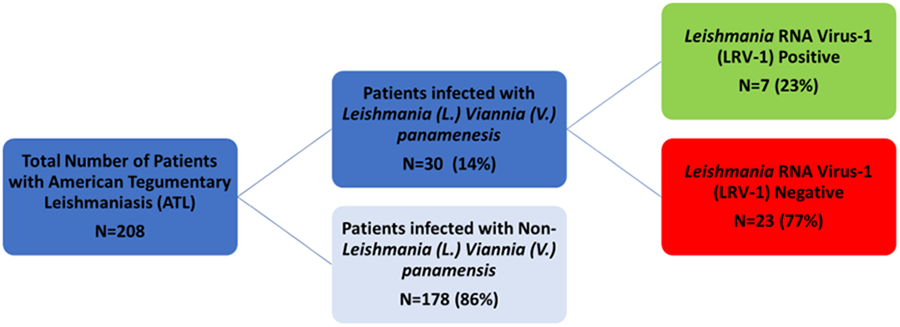
Unique surplus discard clinical specimens of L. V. panamensis were identified from the Public Health Ontario Laboratory (PHOL) and the Leishmania Clinic of the Instituto de Medicina Tropical ‘Alexander von Humboldt’, Lima, Peru between 2012 and 2019 (Fig. 1). Biobanked isolates were confirmed as L. V. panamensis by multiplex real-time polymerase chain reaction (PCR) targeting Leishmania 18S rRNA, following clinical testing, which included microscopic examination of Giemsa-stained smears and/or culture by certified medical lab technologists.

Figure 1. Workflow of sample identification and stratification of patients with confirmed L. (V.) panamensis.
Clinical data
De-identified clinical data of source patients collected from test requisitions and case record forms were stratified into types of ‘severe’ and ‘non-severe’ phenotypes, where a severe phenotype was defined as either mucosal involvement (MCL/ML); or inflammatory ulcers (ulcers with associated erythema, purulent exudate, pain with or without lymphatic involvement) or multifocal/disseminated ulcers (ulcers in ⩾2 anatomic sites and ⩾4 in number) as per the Infectious Diseases Society of America guidelines (Aronson et al., Reference Aronson, Herwaldt, Libman, Pearson, Lopez-Velez, Weina, Carvalho, Ephros, Jeronimo and Magill2016), understanding that the pathogenesis underpinning mucosal vs severe cutaneous manifestations of Leishmania infection are quite different. A non-severe phenotype was defined as LCL of <4 ulcers in number (Aronson et al., Reference Aronson, Herwaldt, Libman, Pearson, Lopez-Velez, Weina, Carvalho, Ephros, Jeronimo and Magill2016).
DNA extraction
DNA extraction was performed using the Qiagen DNA Mini Kit (Qiagen, MD, USA) using 200 μL of cultured specimen with a final elution volume of 60 μL. In the case of primary clinical specimens including filter paper lesion impressions (FPLIs), biopsies, and cytology brushes, specimens were soaked in 200 μL of Tris-EDTA buffer (TE) prior to extraction to achieve sufficient volume and DNA concentration and eluted in 60 μL nuclease-free water.
RNA extraction
RNA was extracted from cultured promastigotes using the Cells Protocol of the QIAamp RNA Mini Kit and eluted with 50 μL of RNase-free water. RNA was extracted from tissue biopsy and cytology brushes using the Fibrous Tissue Protocol from the Qiagen RNeasy Micro Kit with the addition of carrier RNA and eluted with 14 μL RNase-free water. RNA was extracted from FPLIs with the QIAmp RNA Blood Mini Kit and eluted with 30 μL RNase-free water. An in-column DNase treatment was included using the Qiagen rDNase Set as per manufacturer's protocol.
cDNA synthesis and purification
cDNA was performed using 10 μL of RNA in combination with the superscript II reverse transcriptase and random hexamers (Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017). PCR purification was performed using the Qiagen QIAquick PCR Purification Kit and eluted with 60 μL nuclease-free water.
Species identification
Species identification was performed using the following gene targets by end-point PCR: internal transcriber space 1 (ITS1), ITS2, cysteine proteinase B, heat shock protein 70, mannose phosphate isomerase, zinc-dependent metalloproteinase and confirmatory Sanger sequencing (Schonian et al., Reference Schonian, Nasereddin, Dinse, Schweynoch, Schallig, Presber and Jaffe2003; de Almeida et al., Reference de Almeida, Steurer, Koru, Herwaldt, Pieniazek and da Silva2011; Wortmann et al., Reference Wortmann, Sweeney, Houng, Aronson, Stiteler, Jackson and Ockenhouse2011; Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017). Restriction fragment length polymorphism analysis was performed on each product of end-point PCR (de Almeida et al., Reference de Almeida, Steurer, Koru, Herwaldt, Pieniazek and da Silva2011; Wortmann et al., Reference Wortmann, Sweeney, Houng, Aronson, Stiteler, Jackson and Ockenhouse2011; Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017).
Sanger sequencing
Sanger sequencing was performed using 1 μL of PCR product, 2 μL of Big Dye, 3 μL of buffer and 2 μL of 10 μ m of primer and cleaned accordingly (Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017). Products were then centrifuged for 2 min at 2000 g prior to being loaded onto the Applied Biosystems 3730xl DNA Analyser. Data were standardized using the Sequencing Analyser program and BLAST search engine was used to analyse the sequence (Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017).
LRV-1 detection and quantification
LRV-1 was detected in isolates of L. V. panamensis by real-time PCR using 2 primer sets, set A and set B, respectively, as depicted in Fig. 1 (Schmittgen and Livak, Reference Schmittgen and Livak2008; Zangger et al., Reference Zangger, Ronet, Desponds, Kuhlmann, Robinson, Hartley, Prevel, Castiglioni, Pratlong, Bastien, Müller, Parmentier, Saravia, Beverley and Fasel2013; Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017). Leishmania kinetoplastid membrane protein 11 (kmp11) was used as a reference for quantification where sufficient RNA volume for quantification permitted this analysis (Tarr et al., Reference Tarr, Aline, Smiley, Scholler, Keithly and Stuart1988; Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017). Each isolate was run in triplicate and contained the L. V. guyanensis ATCC® (American Type Culture Collection®) 50126™ (MHOM/BR/75/M4147) positive control to perform relative quantification using the 2 − ΔΔCt method (Ogg et al., Reference Ogg, Carrion, de Carvalho Botelho, Mayrink, Correa-Oliveira and Patterson2003; Schmittgen and Livak, Reference Schmittgen and Livak2008; Zangger et al., Reference Zangger, Ronet, Desponds, Kuhlmann, Robinson, Hartley, Prevel, Castiglioni, Pratlong, Bastien, Müller, Parmentier, Saravia, Beverley and Fasel2013; Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017). If kmp11 was not detected, a pre-amplification step was performed as per Perfecta Pre-Amp Supermix guidelines. In the case that kmp11 remained undetected after pre-amplification, the 18S rRNA gene was used as a reference and a relative quantification was performed using the 2 − ΔΔCt method (Wortmann et al., Reference Wortmann, Sweeney, Houng, Aronson, Stiteler, Jackson and Ockenhouse2011; Schmittgen and Livak, Reference Schmittgen and Livak2008; de Almeida et al., Reference de Almeida, Steurer, Koru, Herwaldt, Pieniazek and da Silva2011; Zangger et al., Reference Zangger, Ronet, Desponds, Kuhlmann, Robinson, Hartley, Prevel, Castiglioni, Pratlong, Bastien, Müller, Parmentier, Saravia, Beverley and Fasel2013; Bourreau et al., Reference Bourreau, Ginouve, Prevot, Hartley, Gangneux, Robert-Gangneux, Dufour, Marie, Bertolotti, Pratlong, Martin, Schutz, Couppie, Fasel and Ronet2016; Kariyawasam et al., Reference Kariyawasam, Grewal, Lau, Purssell, Valencia, Llanos-Cuentas and Boggild2017; Schonian et al., Reference Schonian, Nasereddin, Dinse, Schweynoch, Schallig, Presber and Jaffe2003).
Analysis
Descriptive statistics (proportions, mean with s.d., median, range) were calculated for all variables. Differences between categorical variables were compared using Fisher's exact test or χ 2 analysis. Continuous variables were compared by Kruskal–Wallis test or Student's t-test. Significance was set at P < 0.05. Data were analysed using GraphPad Prism (GraphPad, CA, USA).
Results
Clinical and demographic data
Of 208 specimens from patients with confirmed ATL, 30 (14.4%) isolates were identified as L. V. panamensis (Fig. 1). Demographic and parasitological factors for the 30 L. V. panamensis isolates from patients with ATL enrolled and analysed are summarized in Table 1. Eighteen (60%) patients were male, while 12 (40%) were female and the median age was 35 years (range 9–80 years). Sixteen (53.3%) isolates were derived from patients with LCL, while 14 (46.7%) were from patients with inflammatory/multifocal CL and zero (0%) patients with MCL/ML. Fourteen (47%), 7 (23%) and 4 (13%) had travel history to or resided in: Costa Rica, Peru and Ecuador, respectively.
Table 1. Demographic data for 30 patients with L. (V.) panamensis isolates by clinical phenotype

LRV-1 prevalence and copy number by phenotype: primary outcome
A total of 7/30 (23%) isolates contained LRV-1 while 23/30 (77%) did not. Five of 14 (36%) isolates of patients with inflammatory/multifocal phenotypes were LRV-1-positive while 2/16 (12%) isolates from patients with the non-severe phenotype were LRV-1-positive (Table 1).
Clinical phenotype and LRV-1 prevalence by demographics: secondary outcomes
Median ages of patients were distributed across phenotypes as follows: 35 years (range 9–80 years) for those with inflammatory/multifocal CL and 34.5 years (range 17–64 years) for those with LCL, respectively (P = 0.17) (Table 1). One (50%) child had an inflammatory/multifocal phenotype (n = 2); 9 (35%) individuals in the 18–65 years age bracket manifested inflammatory/multifocal CL (n = 26), while those >65 (100%) exclusively manifested the inflammatory/multifocal CL phenotype (n = 4). Male sex (n = 65/78) was distributed across phenotypes as follows: 33% (n = 6/18) with inflammatory/multifocal CL and 67% (12/18) with LCL (P = 0.17). Twelve females were included in the analysis, of which 8 (67%) had the inflammatory/multifocal and 4 (33%) had the LCL phenotypes, respectively.
Median age of patients whose isolates were LRV-1-positive and caused inflammatory/multifocal CL and LCL were: 35 years (range 9–80 years) and 35 years (range 17–80 years), respectively (P = 0.91). LRV-1 positivity was not associated with median age, whereby patients whose isolates were LRV-1-positive had a median age of 35 years (range 9–71 years) compared to LRV-1-negative patients whose median age was 35 years (range 17–80 years) (P = 0.91). However, LRV-1 positivity was detected in only 1 (25%) isolate from patients >65 years (n = 4); 5 (21%) isolates from patients aged 18–65 years (n = 24); and 1 (50%) isolate from patients <18 years (n = 2) (P = 0.21).
LRV-1 copy number
Relative LRV-1 copy number was calculated for 3/7 (43%) isolates positive for LRV-1. The mean relative copy number was identified in 3 isolates from patients with the inflammatory/multifocal phenotype that was 1.09 × 10−4 ± 1.06 × 10−3 (median 1.09 × 10−3, range 6.029 × 10−6 to 2.2 copies).
Discussion
Severity of ATL has been hypothesized to be associated with the viral endosymbiont LRV-1 for decades, with the first report of LRV-1 isolated from a human with cutaneous satellite lesions and lymphatic involvement after visiting Suriname (Tarr et al., Reference Tarr, Aline, Smiley, Scholler, Keithly and Stuart1988). Since this initial report, there have been significant advancements and availability of molecular diagnostic tools to further investigate and understand the role of LRV-1 in ATL, and further accrual of data in humans (Ogg et al., Reference Ogg, Carrion, de Carvalho Botelho, Mayrink, Correa-Oliveira and Patterson2003; Pereira et al., Reference Pereira, Maretti-Mira, Rodrigues, Lima, Oliveira-Neto, Cupolillo, Pirmez and Oliveira2013; Valencia et al., Reference Valencia, Adaui, Chantry, Alba, Ramos, Arevalo, Llanos-Cuentas and Boggild2014; Cantanhede et al., Reference Cantanhede, Silva, Ito, Felipin, Nicolete, Salcedo, Porrozzi, Cupolillo and Ferreira2015; Ito et al., Reference Ito, Catanhede, Katsuragawa, Silva, Camargo, Mattos and Vilallobos-Salcedo2015; Adaui et al., Reference Adaui, Lye, Akopysants, Zimic, Llanos-Cuentas, Garcia, Maes, Doncker, Dobson, Arevalo, Dujardin and Beverley2016; Bourreau et al., Reference Bourreau, Ginouve, Prevot, Hartley, Gangneux, Robert-Gangneux, Dufour, Marie, Bertolotti, Pratlong, Martin, Schutz, Couppie, Fasel and Ronet2016; Ginouves et al., Reference Ginouves, Simon, Bourreau, Lacoste, Ronet, Couppie, Nacher, Demar and Prevot2016; Macedo et al., Reference Macedo, Menezes-Neto, Rugani, Rocha, Silva, Melo, Lye, Beverley, Gontijo and Soares2016). It has been shown that LRV-1 and Leishmania parasites have co-evolved with clustering of both the virus and the parasite in specific geographic locations. Given the species-specific and geographic correlates of observed phenotype in ATL, LRV-1 has the potential to contribute to the diagnosis, treatment and prognostic decision-making in the care of ATL patients (Cantanhede et al., Reference Cantanhede, Fernandes, Ferreira, Porrozzi, Ferreira and Cupolillo2018). Using clinical strains of L. V. panamensis in this study, we examined the overall prevalence of LRV-1 and its possible correlation to clinical phenotypes in a species previously not recorded to contain the virus. While no direct relationship between LRV-1 positivity and negativity with 2 discrete phenotypes was observed, only patients manifesting inflammatory/multifocal CL had a quantifiable viral load.
By analysing LRV-1 status in 30 isolates of L. V. panamensis causing various clinical phenotypes of ATL, an overall 23% prevalence was identified, which is within the range reported previously from studies of strains in Latin America, specifically in L. V. guyanensis and L. V. braziliensis (Salinas et al., Reference Salinas, Zamonra, Stuart and Saravia1996; Wortmann et al., Reference Wortmann, Sweeney, Houng, Aronson, Stiteler, Jackson and Ockenhouse2011; Pereira et al., Reference Pereira, Maretti-Mira, Rodrigues, Lima, Oliveira-Neto, Cupolillo, Pirmez and Oliveira2013; Cantanhede et al., Reference Cantanhede, Silva, Ito, Felipin, Nicolete, Salcedo, Porrozzi, Cupolillo and Ferreira2015; Ito et al., Reference Ito, Catanhede, Katsuragawa, Silva, Camargo, Mattos and Vilallobos-Salcedo2015; Adaui et al., Reference Adaui, Lye, Akopysants, Zimic, Llanos-Cuentas, Garcia, Maes, Doncker, Dobson, Arevalo, Dujardin and Beverley2016; Macedo et al., Reference Macedo, Menezes-Neto, Rugani, Rocha, Silva, Melo, Lye, Beverley, Gontijo and Soares2016). LRV-1 has been loosely described in other species, particularly L. amazonensis and L. naiffi. It has been shown that LRV-1 is not preferentially associated with a specific phenotype (Adaui et al., Reference Adaui, Lye, Akopysants, Zimic, Llanos-Cuentas, Garcia, Maes, Doncker, Dobson, Arevalo, Dujardin and Beverley2016), although this study identified 36% inflammatory/multifocal CL patients were LRV-1-positive compared to 12% of LCL patients with no patients being identified with ML/MCL in this population. While these proportions were not statistically different, it is possible that with a larger-scale prospective study, a meaningful difference in the LRV-1 prevalence could emerge. Furthermore, it is possible to understand if LRV-1 in L. V. panamensis contributes to the ML/MCL phenotype, however this has not been documented in literature. The relationship of both LRV-1 prevalence and viral burden to clinical manifestations and observed phenotype warrants additional work in larger cohort of patients with ATL, specifically in patients with inflammatory/multifocal CL.
Although there was no age difference observed in LRV-1 isolates, the detection of the virus was documented in areas of Central America including Costa Rica, Belize and Panama, where LRV-1 has historically not been detected. MCL/ML was not identified in this cohort of L. V. panamensis, perhaps in this patient population, inflammatory/multifocal CL is considered the most severe phenotype achievable in this species, given that LCL was restricted to patients <65 years of age. Perhaps there is progression to inflammatory/multifocal CL after LCL (Reithinger et al., Reference Reithinger, Dujardin, Louzir, Pirmez, Alexander and Brooker2007; Jara et al., Reference Jara, Valencia, Adaui, Alba, Lau, Arevalo, Llanos-Cuentas and Boggild2016). One possible explanation for why LRV-1 may be less likely to occur in older patients who are from endemic settings is the recurrent, lifelong exposure, which could enable the parasite to harness the endogenous RNAi activity of the Viannia subgenus to eliminate the virus over time (Brettman et al., Reference Brettman, Shaik, Zangger, Lye, Kuhlmann, Akopyants, Oschwald, Owens, Hickerson, Ronet, Fasel and Beverley2016). In this study, all but 1 isolate from patients over age 65 (n = 4) were found to be LRV-1-positive, and 1 isolate from patients under age 18 was LRV-1-positive. Advanced age is associated with poorer T-cell response and a Th2-biased response, in particular (Salam et al., Reference Salam, Rane, Das, Faulkner, Gund, Kandpal, Lewis, Mattoo, Prabhi, Ranganathan, Durdik, George, Rath and Bal2013), which in the case of ATL, is correlated to poorer immunologic control of infection and persistence of the amastigote in the phagolysosome (Hartlet et al., Reference Hartlet, Ronet, Zangger, Beverley and Fasel2012). Similarly, the Th1-to-Th2 ratio has been demonstrated to be lowest in childhood and adolescence, with a peak during mid-adulthood, and slight decline thereafter (Chang et al., Reference Chang, Kim, Lim, Yoon, Son, Park, Hong, Cho and Lee2016). Th2 predominance over Th1 is also an important factor in the progression of severe disease (Tripathi et al., Reference Tripathi, Singh and Naik2007; Hartley et al., Reference Hartley, Kohl, Ronet and Fasel2013; Maspi et al., Reference Maspi, Abdoli and Ghaffarifar2016; Moafi et al., Reference Moafi, Rezvan, Sherkat, Taleban, Asilian, Zarkesh-Esfahani, Nilforoushzadeh, Jaffary, Mansourian, Sokhanvari and Ansari2017). Understanding the potential behavioural, socioeconomic and biological underpinnings of the age distributions of LRV-1 noted in this analysis will be, ultimately, important to accurate interpretation of the viral role in ATL pathogenesis.
Limitations of this descriptive analysis of LRV-1 prevalence amongst L. V. panamensis isolates include the comparatively small number of isolates, as well as enrolment from patients who are returning travellers from Latin America to Canada or live in Peru, with a majority of travellers having gone to Costa Rica. Prospective enrolment of larger cohorts that might enable more even distribution of returning travellers would be worthwhile. It is also possible that significantly different proportions of LRV-1 positivity by phenotype might have emerged with a larger cohort. While a limited budget did not permit such a large-scale analysis, the findings are important as, even in this smaller cohort, the presence of LRV-1 in a species with very limited literature and higher viral load in L. V. panamensis isolates causing inflammatory/multifocal CL, also suggests some interesting age preponderances that will be best interrogated using a combination of epidemiologic and basic scientific approaches going forward.
Conclusions
Continued exploration of LRV-1 prevalence across age groups, particularly in larger cohorts, with specific interrogation of immunological age correlates of LRV-1-positivity while controlling for behavioural, socioeconomic and other possible biological contributors to the age biases observed herein, will be essential to understanding the relevance of this demographic variable to the host–parasite–viral interplay that governs phenotype. The role of LRV-1 as a predictive biomarker of disease severity remains unclear, however the mechanistic nature, particularly regarding the immune response, will prove useful to understanding overall ATL-LRV-1 pathogenesis particularly in patients with inflammatory/multifocal CL.
Data availability statement
All data and other materials necessary are included in the article.
Author contributions
R. K. contributed to study design; data collection, analysis and interpretation; and was primarily responsible for drafting the manuscript. R. L. contributed to study design; data collection, analysis and interpretation; and to manuscript revision and critical appraisal. B. M. V. and A. L.-C. contributed to study design; data collection and interpretation; and to manuscript revision and critical appraisal. A. K. B. conceived the study and contributed to study design; funding acquisition; data collection, analysis and interpretation; and to writing and revising subsequent iterations of the manuscript. All authors serve as guarantors of the work.
Financial support
This work was supported by Public Health Ontario via the Project Initiation Fund and the University of Toronto via an Early Career Department of Medicine award. Dr Boggild is supported as a Clinician Scientist by the Departments of Medicine at the University of Toronto and University Health Network.
Competing interests
None.
Ethical standards
Approval for this study was obtained from the Ethics Review Board of Public Health Ontario, the Research Ethics Board of University of Toronto and the Institutional Review Board of Hospital Nacional Cayetano Heredia, Lima, Peru.