Introduction
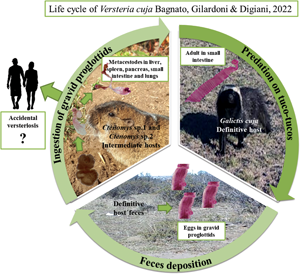
Taeniid species have a worldwide distribution and are of medical and veterinary importance because adult tapeworms are responsible for intestinal infections, whereas metacestodes are responsible for systemic infections in domestic and wild animals, and in humans (Deplazes et al., Reference Deplazes, Eichenberger and Grimm2019). In taeniid life cycles, members of the order Carnivora mainly act as definitive hosts whereas their prey, largely belonging to the orders Rodentia, Lagomorpha and Artiodactyla, act as intermediate hosts (Loos-Frank, Reference Loos-Frank2000; Hoberg, Reference Hoberg2006). Within the family, the genus Versteria Nakao, Lavikainen, Iwaki, Konyaev, Oku, Okamoto and Ito, 2013 includes 3 species: Versteria mustelae (Gmelin, 1790) (type-species), with an Holarctic distribution; Versteria brachyacantha (Baer and Fain, 1951) described from Africa (current Rwanda); and Versteria cuja Bagnato, Gilardoni and Digiani, Reference Bagnato, Gilardoni, Martin and Digiani2022 from Argentina. Recent studies suggested that: (1) V. mustelae could represent a species complex, in which at least 4 different lineages can be identified (Western Europe, Siberia /Japan, China and USA), and (2) there are 2 different lineages of Versteria in the Nearctic: one related to the Palearctic V. mustelae and another one, not yet formally described but characterized molecularly, known as ‘zoonotic’ Versteria sp. and responsible for fatal and/or severe infections by metacestodes in wildlife, captive primates and immunosuppressed people (Goldberg et al., Reference Goldberg, Gendron-Fitzpatrick, Deering, Wallace, Clyde, Lauck, Rosen, Bennett, Greiner and O'Connor2014; Lee et al., Reference Lee, Wallace, Clyde, Gendron-Fitzpatrick, Sibley, Stuchin, Lauck, O'Connor, Nakao, Lavikainen, Hoberg and Goldberg2016; Deplazes et al., Reference Deplazes, Eichenberger and Grimm2019; Niedringhaus et al., Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021). Some of the older reports of Taenia mustelae from North America (e.g. Skinker, Reference Skinker1935; Locker, Reference Locker1955; Freeman, Reference Freeman1956) could be either species.
Versteria cuja seems to be related to the Nearctic Versteria sp. (Bagnato et al., Reference Bagnato, Gilardoni, Martin and Digiani2022) and the conspecificity of both taxa cannot be ruled out. Consequently, V. cuja is of importance in view of its zoonotic potential in this region. Versteria cuja uses the lesser grison, Galictis cuja (Molina) as definitive host, a native mustelid which occupies a wide variety of habitats (Larivière and Jennings, Reference Larivière, Jennings, Mittermeir and Wilson2009), and whose diet is mostly composed of small to medium sized vertebrates (e.g. rodents, hares) (Chébez et al., Reference Chébez, Pardiñas and Teta2014). Particularly in Chubut province (Argentina), near Esquel and Trevelin cities (the type locality of V. cuja), the lesser grisons mainly feed on rodents, including tuco-tucos Ctenomys Blainville (Rodentia: Ctenomyidae) (Delibes et al., Reference Delibes, Travaini, Zapata and Palomares2003; G. M. Martin, pers. obs.). In the neighbourhood of the type locality of V. cuja, and in the frame of a study conducted by mammalogists (Doctoral Fellowship project of F. Brook), 37 specimens of tuco-tucos of different species were captured and examined for parasites. Two of the rodents examined (Ctenomys sp. 1 and Ctenomys sp. 2) harboured metacestodes in different organs. Molecular analyses conducted on these metacestodes corroborated their conspecificity with the adults of V. cuja found in lesser grisons in the type locality. The aim of this paper is to shed light on the 2-host life cycle of V. cuja, providing a morphological and molecular characterization of the metacestodes found, as well as a histopathological description of the metacestodiasis affecting the liver and the lungs of the intermediate hosts.
Materials and methods
Study area and sample collection
Four individuals of Ctenomys sp. 1 were collected near Terraplén Lagoon (42.96°S, 71.49°W) in April 2019, Chubut province, Argentina. The habitat is a transitional environment between the Subantarctic beech-tree forest to the west and the Patagonian steppe to the east. One individual of Ctenomys sp. 2 was collected near Talagapa (42.16°S, 68.17°W) (Patagonian steppe) in November 2021. The names ‘Ctenomys sp. 1’ and ‘Ctenomys sp. 2’ represent 2 different, undescribed species, studied as part of the Doctoral work of F. Brook and still unpublished. One specimen of Ctenomys haigi Thomas collected near Esquel Airport ‘Brigadier General Antonio Parodi’ (42.87°S, 71.13°W) in March 2022 was available for histological comparison of normal liver tissue. All captures were made under permits provided by the Dirección Fauna y Flora Silvestre from the Ministerio de Agricultura, Ganadería, Industria y Comercio del Chubut (Resolutions N° 1468/2019, N° 404/2021). Specimens were caught using Oneida Victor N° 0 traps with rubber covers and were euthanized by cervical dislocation (Sikes et al., Reference Sikes and Gannon2011; Brook et al., Reference Brook, Tomasco, González and Martin2022). Tuco-tucos from Terraplén Lagoon were necropsied fresh and the Ctenomys sp. 2 from Talagapa was fixed in 96% ethanol before examination. All of them were inspected for endoparasites under a Leica EZ4 stereomicroscope (Leica, Wetzlar, Germany). The gastrointestinal tract was separated into oesophagus, stomach, caecum and intestine. The body cavity, liver, pancreas, spleen, gall bladder, gonads, lungs, heart and kidneys were also examined for parasites.
Parasitological study
Most metacestodes from Ctenomys sp. 1 were fixed in 4% formalin/distilled water (after washing with physiological solution), and preserved and stored in 70% ethanol; some others were stored directly in absolute ethanol for molecular analysis. Metacestodes from Ctenomys sp. 2 were fixed and preserved in 96% ethanol. Specimens designated for morphological study were stained with Semichon's acetic carmine orLangeron's carmine, dehydrated through an ethanol series, cleared in eugenol and mounted in Canada balsam for examination under a light microscope Leica DM500 (Leica, Wetzlar, Germany). To observe the rostellum and rostellar hooks in detail, rostella from several metacestodes were dissected and mounted between slides and coverslips in Hoyer's fluid and allowed to dry. Measurements and photographs were taken with a Leica ICC50W camera with software connected to the microscope. Measurements are given in micrometres (μm) as range, followed by mean in parentheses, unless otherwise stated. Prevalence and mean intensity of metacestodes infection in Ctenomys sp. 1 were calculated following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Mounted vouchers of metacestodes of V. cuja were deposited in the Helminthological Collection of the Museo de La Plata (MLP-He), La Plata, Argentina. Specimens of Ctenomys sp. 1, Ctenomys sp. 2 and C. haigi were deposited in the Mammal Collection of the Laboratorio de Investigaciones en Evolución y Biodiversidad (LIEB-M), Esquel, Argentina.
Statistical analysis
A 1-way analysis of variance was performed (factor: intermediate host species) using the R software and plyr package (R Core Team, 2023) in R Studio (RStudio Team, 2023) for summary measurements of the metacestodes (mean ± standard error), shown in Table 1.
Table 1. Comparison of some measured characters (in micrometres) of Versteria cuja metacestodes (mono- and polycephalic forms) between Ctenomys sp. 1 from Terraplén Lagoon and Ctenomys sp. 2 from Talagapa, Chubut province, Argentina, using one-way analysis of variance (ANOVA)

s.e., standard error.
Asterisks for P levels (*P < 0.05, **P < 0.01, ***P < 0.001).
DNA extraction, amplification and sequencing
Eight sequences were generated from a mitochondrial DNA region, cytochrome c oxidase subunit 1 gene (cox1) extracted from 2 cysticerci from the liver of Ctenomys sp. 1 (forward and reverse) of the liver from Terraplén Lagoon and, 2 polycephalic larvae, 1 from the liver and 1 from the pancreas (forward and reverse) of Ctenomys sp. 2 from Talagapa, each using the Wizard® Genomic DNA Purification Kit (A1120) following the manufacturer's instructions. The amplification and sequencing procedure follows Bagnato et al. (Reference Bagnato, Gilardoni, Martin and Digiani2022). Sequences were deposited in GenBank. Sequences from other Versteria species as well as other species of Taeniidae were taken from GenBank and compared using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Sequence alignment
Sequences obtained from cysticerci from Ctenomys sp. 1 and Ctenomys sp. 2 were aligned and compared with the cox1 sequences obtained from adults of V. cuja using Multalin software (available at http://www.sacs.ucsf.edu/cgi-bin/multalin.py).
Histological study
A liver sample from Ctenomys sp. 1 and cysticerci were fixed in 4% formalin and preserved in 70% ethanol; the liver was separated for histology and cysticerci for definitive preparations. A lung sample from Ctenomys sp. 2 was separated for histology. Liver samples from Ctenomys sp. 1 and C. haigi, and lung samples from Ctenomys sp. 2 were dehydrated, embedded in paraffin and sectioned at 3–5 μm following standard histological techniques. Sections were stained with haematoxylin–eosin (H&E), toluidine blue and Gomori's trichrome for morphological description. Other liver sections from Ctenomys sp. 1 were incubated separately with the primary antibodies pancytokeratin (Pk, dilution 1/250, Agilent, Z0622) to identify epithelial origin cells, and proliferating cell nuclear antigen (PCNA, 1/3000 dilution, Sigma-Aldrich, SAB2701819) to identify proliferating cells. All techniques used were based on standardized protocols of the Laboratorio de Histología y Embriología Descriptiva, Experimental y Comparada, Facultad de Ciencias Veterinarias, Universidad Nacional de La Plata (Acuña et al., Reference Acuña, Barbeito, Portiansky, Miglino and Flamini2021).
Results
Cyclophyllidea van Beneden in Braun, 1900
Taeniidae Ludwig, 1886
Versteria cuja Bagnato, Gilardoni and Digiani, 2022
Description of metacestodes
Both mono- and polycephalic larvae were found in both intermediate hosts infecting several organs (Fig. 1). Both types of metacestodes exhibited a great variation in shapes (Fig. 2). The primary morphological characters allowing the association of these metacestodes to V. cuja were the rostellar hooks (number, size and shape); these are fully formed and represented by 3 typical parts: handle, blade and guard. The general measurements of 10 complete hooks of metacestodes from both intermediate hosts are: 13 (10–16) in total length, 8 (6–10) maximum width, handle 7 (5–10) in length by 4 (3–5) width, blade 6 (5–8) in length by 2 (2–3) width and guard 7 (5–8) in length by 3 (2–4) width (Fig. 3). Hooks are 40–48 in number, arranged in 2 alternating rows (Fig. 3).

Fig. 1. (A, B). Metacestodes of Versteria cuja Bagnato, Gilardoni and Digiani, Reference Bagnato, Gilardoni, Martin and Digiani2022 (Cestoda: Taeniidae) invading organs in Ctenomys sp. 1 from Terraplén Lagoon, Chubut, Argentina. (A) Cysticercosis in liver, macroscopic view. (B) Mono- and polycephalic forms in small intestine, in fresh. B, bladder; Li, liver; Me, metacestodes; MF-Cy, monocephalic form-cysticercus; MF-E, monocephalic form-evaginated; PF, polycephalic forms; SI, small intestine.

Fig. 2. (A–J). Multiplicity of shapes and sizes of metacestodes of Versteria cuja Bagnato, Gilardoni and Digiani, Reference Bagnato, Gilardoni, Martin and Digiani2022 (Cestoda: Taeniidae) found in Ctenomys sp. 1 from Terraplén Lagoon and Ctenomys sp. 2 from Talagapa, Chubut, Argentina. (A–H, M, N) Monocephalic forms. (I–L, O–S) Polycephalic forms. Note some forms with evaginated scoleces (C, D, H, I, J, L). (A–D, N, O–S) from the liver; (E, F) from the spleen; (G–L) from the small intestine; (M) from the pancreas. Stain: Langeron's carmine.

Fig. 3. (A–J). Rostella and rostellar hooks from metacestodes of Versteria cuja Bagnato, Gilardoni and Digiani, Reference Bagnato, Gilardoni, Martin and Digiani2022 (Cestoda: Taeniidae) found in Ctenomys sp. 1 and Ctenomys sp. 2 from Chubut, Argentina. (A–F) Rostella, showing the 2 crowns of alternate rostellar hooks of similar size, in different views, Hoyer's fluid. (G, H) Rostellar hooks, showing the 3 parts (handle, blade and guard) and measurements taken, lactoglycerol. (I, J) Rostellar hooks in histological sections, H&E. Bl, blade; BL, blade length; BW, blade width; Gu, guard; GL, guard length; GW, guard width; H, hooks, Ha, handle; HL, handle length; HW, handle width; MW, maximum width; R, rostellum; S, sucker; TL, total length.
Monocephalic larvae (Figs 1–3, Table 1, Supplementary Tables S1 and S2)
These forms corresponded to the cysticercus type, with one scolex per bladder. They were found mainly invading the liver, occupying the entire organ both superficially and deeply. Cysticerci also invaded the spleen, pancreas, small intestine and lungs, but in smaller numbers. Most larvae encountered had the typical cysticercus appearance with one scolex invaginated into the bladder. Some forms, however, showed evaginated scoleces. All scoleces observed exhibited 4 unarmed suckers and a rostellum bearing 2 rows of small hooks (Fig. 3).
Measurements provided are based on 66 metacestodes from different organs from both species of intermediate hosts (Supplementary Tables S1 and S2).
In Ctenomys sp. 1, cysticerci were found in the liver, spleen and small intestine and exhibited different shapes and sizes according to the organ infected. Those from the liver and spleen were smaller than those from the small intestine, which were the largest and had elongated bladders. The largest cysticercus was 8 mm in length by 2.75 mm wide (from the small intestine) and the smallest one (from the liver) measured 1.25 mm in length by 0.41 mm wide (Supplementary Table S1). Each cysticercus was characterized by a thin, transparent or whitish wall enclosing a fluid-filled bladder and a single small invaginated scolex. In mounted preparations, the bladders were collapsed.
In Ctenomys sp. 2, cysticerci were found in the liver, pancreas and lungs, enclosed in larger cysts containing 1–15 cysticerci. The cysticerci from the pancreas were smaller than those from the liver, which were the largest and had larger bladders. The largest cysticercus was 5.38 mm in length by 2.2 mm wide (from the liver) and the smallest one (from the pancreas) 2.38 in length by 0.88 mm wide (Supplementary Table S2). Each cysticercus had a thin, transparent or whitish wall enclosing a fluid-filled bladder and a single small invaginated scolex. The few larvae identified in the lungs were observed only in the histological sections.
Polycephalic larvae (Figs 1–3, Table 1, Supplementary Table S3)
Polycephalic larvae were found invading the first portion of the small intestine in Ctenomys sp. 1, and the liver and pancreas in Ctenomys sp. 2, the latter enclosed in cysts. Each cyst consisted of a capsule (probably originating from the connective tissue of the host) containing up to 15 larvae. The larvae from the small intestine were not observed inside a cyst; when the intestinal wall was opened, they all came out together in the Petri dish (see Fig. 1B). Polycephalic larvae presented a common bladder filled with fluid and containing a mean of 3 scoleces per bladder (Table 1). Different morphologies were observed: (1) dendritic or branched forms with a small to very small central bladder from which elongate stalks arose, each one bearing a scolex at the tip (mostly invaginated but sometimes evaginated) (Fig. 2I, J, O); (2) forms with a larger, common bladder, from which exogenous buds arose, each one bearing a scolex (Fig. 2K, L, P–S). In general, the mean length was higher in the metacestodes from Ctenomys sp. 1 (Table 1). The largest polycephalic metacestode from Ctenomys sp. 1 was 13.9 mm in length by 5.2 mm wide and the smallest one 3.0 mm in length by 0.9 mm wide. The largest form from Ctenomys sp. 2 was 9.23 mm in length by 6.75 mm wide (from the liver) and the smallest one (from the pancreas) 1.6 mm in length by 1.9 mm wide (Supplementary Table S3). The largest larva has a maximum of 5 scoleces arising from a central bladder.
Comparison of measurements between larvae from the 2 host species did not yield significant differences in diagnostic characters (scolex, rostellum, suckers); as there were few measurements of the different parts of the rostellar hooks, they could not be included in the ANOVA, but no differences were observed between both intermediate hosts. Only slight differences were found in the total larval length, length of the invaginated part (‘neck length’) and length of the exogenous buds in polycephalic larvae, differences which can probably be attributed to differences in the fixation method and to diverse degrees of regression of the central bladder, respectively.
Taxonomic summary
Intermediate hosts: Tuco-tucos, Ctenomys sp. 1 and Ctenomys sp. 2 (Rodentia: Ctenomyidae).
Site of infection and abundance: In Ctenomys sp. 1: mainly in the liver (n = + 150), spleen (n = 8) and small intestine (n = 52). In Ctenomys sp. 2: liver (n = 15), pancreas (n = 30), lungs (n = 3, observed in histological sections).
Prevalence and intensity of infection: In Ctenomys sp. 1, P = 25% (1 out of 4 Ctenomys sp. 1 infected with +158 mono- and 52 polycephalic metacestodes). In Ctenomys sp. 2, one tuco-tuco with at least 48 mono- and polycephalic metacestodes.
Localities: Terraplén Lagoon (42.96°S, 71.49°W), near Los Alerces National Park, Chubut province, Argentina. Talagapa town (42.16°S, 68.17°W), Chubut province, Argentina.
Material deposited: From Ctenomys sp. 1: monocephalic and polycephalic forms, MLP-He 7988. From Ctenomys sp. 2: monocephalic and polycephalic forms, MLP-He 7989.
Host specimens deposited: LIEB-M-1727 (Ctenomys sp. 1, Terraplén Lagoon), LIEB-M-1763 (Ctenomys sp. 2, Talagapa).
Molecular analysis
PCR amplification of cox1, mtDNA from cysticerci of Versteria from the liver of Ctenomys sp. 1 gave 2 products of 376 and 361 bp, respectively; and cysticerci of Versteria from the liver and pancreas of Ctenomys sp. 2 gave 2 products of 313 and 374 bp, respectively, of partial cox1 sequence. The cox1 sequences of cysticerci from Ctenomys sp. 1, cysticerci from Ctenomys sp. 2 and the cox1 sequences of the adult worm from G. cuja (OL345569, OL345573 and OL345572) showed 100% similarity.
GenBank accession numbers: ON980784 and OP379709 (cox1, monocephalic forms from the liver of Ctenomys sp. 1); OP379295 (cox1, polycephalic form from the liver of Ctenomys sp. 2), OP379710 (cox1, polycephalic form from the pancreas of Ctenomys sp. 2).

Fig. 4. (A–D). Histological sections of Ctenomys sp.1 liver infected with Versteria cuja Bagnato, Gilardoni and Digiani, Reference Bagnato, Gilardoni, Martin and Digiani2022. (A) Liver morphology altered by cysticerci. Congested blood vessels, hepatocytes lacking radial arrangement and dilated sinusoidal spaces are seen, haematoxylin–eosin (H&E). (B) Cysticercus surrounded by acidophilic capsule of connective tissue. Next to this area, infiltrate of immune cells: lymphocytes, macrophages and eosinophils. Hepatocytes adjacent to infiltrate and capsule are atrophied. Detail of the sinusoidal dilatation is also seen, H&E. (C) Detail of capsule circumscribing the cysticerci. Adjacent atrophied hepatocytes are observed. Other hepatocytes not atrophied although with degenerative changes (turbid tumefaction), Gomori's trichrome. (D) Degenerating hepatocytes with numerous cytoplasmic vacuoles and nuclear figures, H&E. At, atrophied hepatocytes; BV, blood vessels; Ca, capsule; Cys, cysticercus; DH, degenerating hepatocytes; H, hepatocytes; In, infiltrate; SS, sinusoidal spaces.

Fig. 5. (A–D). Histological sections of Ctenomys sp.1 liver infected with Versteria cuja Bagnato, Gilardoni and Digiani, Reference Bagnato, Gilardoni, Martin and Digiani2022. (A.1–A.2) Mast cells (arrows), cytoplasmatic and metachromatic granules are observed, Toluidine blue. (B) Multiple intact bile ducts are observed in the portal spaces, no alterations are observed in the lining epithelium (Pk+) (arrows), immunohistochemistry for pancytokeratin (Pk). (C) Proliferating epithelial cells (arrows) of the bile duct (PCNA+), immunohistochemistry for proliferating cell nuclear antigen (PCNA). (D) Macrophages with endocytosed haemosiderin (circle with black outline) adjacent to the bile ducts, Gomori's trichrome. CC, calcareous corpuscles; Cys, cysticercus; Lu, lumen.
Histopathological description of Ctenomys sp. 1 liver (Figs 4 and 5)
Cysticerci were observed in the hepatic parenchyma of Ctenomys sp. 1. The liver morphology was altered by the presence of cysticerci (Fig. 4A). Each cysticercus compressed the surrounding hepatocytes, altering their radial arrangement and causing atrophy. Congested blood vessels and dilated sinusoidal spaces were also observed (Fig. 4B). Degenerative changes (turbid tumefaction) were observed in the hepatocytes adjacent to the atrophied ones (Fig. 4C and D). Each cysticercus was surrounded by an acidophilic capsule of dense connective tissue, accompanied by an inflammatory infiltrate in which macrophages, lymphocytes and eosinophils predominated (Fig. 4D). In addition to lymphocytes and macrophages, mast cells with metachromatic granules were observed (Fig. 5A1–A2). Multiple bile ducts were observed intact, in the portal spaces, without morphological changes, but with proliferating cells (Pk and PCNA+) (Fig. 5B and C). Inflammatory cells including macrophages with endocytosed haemosiderin were observed adjacent to the bile ducts (Fig. 5D).
Comparing the parasitized liver of Ctenomys sp. 1 against a healthy liver (C. haigi), the latter showed hepatocytes polygonal shaped, acidophilic cytoplasm and 1–2 nuclei, arranged in trabeculae which radiated into the centrilobular vein. An additional difference was found in the number of bile ducts. Although in the healthy liver there was one bile duct per portal space, there was more than one bile duct in the parasitized liver (Fig. 5B).
Histopathological description of Ctenomys sp. 2 lungs
Cysticerci were observed in the lung parenchyma of Ctenomys sp. 2. The lung morphology was altered by the presence of cysticerci (Fig. 6). Each cysticercus was associated with an inflammatory infiltrate and surrounded by a connective tissue capsule (Fig. 6A–C). Several pulmonary alveoli were observed to be dilated. Other pulmonary alveoli contained eosinophilic material, evidence of oedema, in the lumen. Some blood vessels were observed to be hyperaemic (Fig. 6D).

Fig. 6. (A–D). Histological sections of Ctenomys sp. 2 lung infected with Versteria cuja Bagnato, Gilardoni and Digiani, Reference Bagnato, Gilardoni, Martin and Digiani2022. (A) Two cysticerci surrounded by connective tissue capsule and inflammatory infiltrate in the lung parenchyma, haematoxylin–eosin (H&E). (B, C) Magnifications of (A), H&E. (B) Scolex. (C) Posterior end of the cysticercus. (D) Hyperaemic blood vessels, dilated alveoli and alveoli with eosinophilic content, H&E. A-EC, alveoli with eosinophilic content; Ca, capsule; Cys, cysticercus; DA, dilated alveoli; HBV, hyperaemic blood vessels; In, infiltrate.
Discussion
The similarity (in number, size and shape) of the rostellar hooks of the metacestodes found in Ctenomys sp. 1 and Ctenomys sp. 2 to those observed in the adult cestodes from the lesser grison in the same area (Bagnato et al., Reference Bagnato, Gilardoni, Martin and Digiani2022) lead us to suspect that they could be the metacestodes of V. cuja. This similarity could be observed in definitive and transitory preparations as well as in histological sections. Hooks' dimensions of metacestodes were 13 (10–16) in length by 8 (6–10) wide, whereas in the adult tapeworm these were (12–17) in length by (6–10) wide. The total number of hooks was 40–48 in the metacestodes and 48 in the adults (Bagnato et al., Reference Bagnato, Gilardoni, Martin and Digiani2022). Based on this first evidence, we performed the molecular analysis, which finally confirmed the identity of V. cuja in the 2 intermediate host species. The 100% similarity between the cox1 sequences of cysticerci from Ctenomys sp. 1, polycephalic larvae from Ctenomys sp. 2 and the adult worm from the lesser grison allowed confirmation, for V. cuja, of a 2-host life cycle with the subterranean rodents (Ctenomys sp. 1 and Ctenomys sp. 2) as intermediate hosts and the lesser grison G. cuja as definitive host in Chubut province, Argentina. The metacestodes were found invading different organs and showed a multiplicity of shapes and sizes which can be summarized primarily into 2 types: monocephalic forms (the typical cysticercus) with one scolex per bladder, and polycephalic forms in which 2 or more scoleces arose from a common bladder. In addition, within both types most larvae showed typically invaginated scoleces; however, a small proportion of larvae showed evaginated scoleces.
‘Polycephalic’ larvae were defined by Hoberg et al. (Reference Hoberg, Jones, Rausch, Eom and Gardner2000) as larvae in which 2 or more scoleces are borne on elongate stalks that arise by exogenous budding from a central bladder that later regresses (Hoberg et al., Reference Hoberg, Jones, Rausch, Eom and Gardner2000; Chervy, Reference Chervy2002). These larvae were considered to be derived forms with respect to the cysticercus and characteristic of some taeniid species such as Taenia selousi Mettrick, 1962, Taenia endothoracicus (Kirschenblatt, 1948) and Taenia twitchelli Schwartz, 1927 but also of T. mustelae sensu Freeman (Reference Freeman1956) (Hoberg et al., Reference Hoberg, Jones, Rausch, Eom and Gardner2000). Rossin et al. (Reference Rossin, Timi and Hoberg2010) found both mono- and polycephalic larvae that were assigned to Taenia talicei (Dollfus,1960) in the abdominal cavity of 2 Ctenomys species (C. talarum Thomas and C. australis Rusconi) from Buenos Aires province, Argentina (Rossin et al., Reference Rossin, Timi and Hoberg2010).
The multiplicity of forms encountered among the polycephalic larvae of V. cuja could be explained by the different degrees of regression of the central bladder. As mentioned above, most larvae showed typically invaginated scoleces; however, a small proportion showed evaginated scoleces. Moreover, some polycephalic larvae exhibited the 2 conditions simultaneously (see Fig. 2J and L).
The significance of the evagination and invagination of the scolex, at least within the Taeniidae, remains unclear (see Świderski et al., Reference Świderski, Miquel, Młocicki, Georgiev, Eira, Grytner-Ziȩcina and Feliu2007). Even when taeniids are characterized by scolex morphogenesis in an invaginated condition, exceptions may appear. As summarized by Loos-Frank (Reference Loos-Frank2000) and Świderski et al. (Reference Świderski, Miquel, Młocicki, Georgiev, Eira, Grytner-Ziȩcina and Feliu2007) among taeniid species with polycephalic larvae, Taenia crassiceps (Zeder, 1800), T. endothoracicus, T. mustelae, Taenia parva Baer, 1926 and T. twitchelli have post-embryonic larvae with scoleces invaginated. In contrast, evaginated scoleces have been reported in Taenia krepkogorski (Schulz and Landa, 1934) and T. selousi.
On the other hand, the evagination of the scoleces of other taeniid species and genera may be induced in in vitro conditions. For example, Maimaitizunong et al. (Reference Maimaitizunong, Li, Wu, Tian, Qi, Jiao, Zhang, Gong, Guo, Zhang and Zhang2022) demonstrated that a brief stimulation with water (2 times of 20s) prior to in vitro cultivation of protoscoleces of E. granulosus and E. multilocularis increased the evagination process, resulting in 85–90% of evaginated protoscoleces.
Thus, our observations of a few evaginated scoleces among the isolated larvae could be related to the medium in which cysticerci weremanipulated. Cysticerci from Ctenomys sp. 1, which exhibited both invaginated and evaginated scoleces, were washed in physiological solution prior to their fixation in 4% formalin, while cysticerci from Ctenomys sp. 2 (all invaginated forms) were placed directly in 96% ethanol. The presence of one larva with an evaginated scolex surrounded by the host capsule within the lung (Fig. 6) is, on the contrary, more difficult to explain.
The larval stages of V. cuja are largely similar to those described by Freeman (Reference Freeman1956) for ‘Taenia mustelae’ from the USA in naturally and experimentally infected intermediate hosts. Freeman (Reference Freeman1956) also found infection in various organs, and monocephalic and polycephalic forms coexisting in a same individual host. The rostellar hooks of the metacestodes of V. cuja had similar shape and size than those of the North American species, having a short and sharply curved blade; short, straight to sinuous handle and long stout guard. The handle ended in a stout bulb and the posterior edge was frequently folded, in a similar way to that described by Freeman (Reference Freeman1956). The hooks of metacestodes of V. cuja ranged in total length from 10 to 16 vs 14 to 20 in those studied by Freeman (Reference Freeman1956).
It is worth noting that studies on the life cycle of V. mustelae (=T. mustelae) in the Palaearctic region (Murai, Reference Murai1981; Iwaki et al., Reference Iwaki, Abe, Shibahara, Oku and Kamiya1996) reported only monocephalic cysticerci, whereas in the Nearctic ‘T. mustelae’ (as it appears in older reports) mono- and polycephalic metacestodes simultaneously occur (Loos-Frank, Reference Loos-Frank2000). It seems then that a life cycle with mono- and polycephalic larvae is another feature characterizing a distinct Nearctic lineage of Versteria (different from V. mustelae), besides the molecular differences and the zoonotic potential (Skinker, Reference Skinker1935; Locker, Reference Locker1955; Freeman, Reference Freeman1956; McKeever and Henry, Reference McKeever and Henry1971; Niedringhaus et al., Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021).
Indeed, Niedringhaus et al. (Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021) described a case of fatal infection by Versteria in a muskrat Ondatra zibethicus from Pennsylvania, USA. Multiple organs of the muskrat were infected, harbouring dozens of cysts lined by a thin, fibrous capsule and containing solid-bodied larvae with up to 4 invaginated, small scoleces bearing short rostellar hooks (up to 11 in length), arranged in 2 rows. These larvae were named by Niedringhaus et al. (Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021) as ‘polycephalic cysticerci’. Its genetic characterization confirmed the cestode as belonging to the lineage known as ‘zoonotic’ Versteria sp., known from several cases of metacestodiasis in humans and other primates, probably caused by the accidental ingestion of the eggs (see Deplazes et al., Reference Deplazes, Eichenberger and Grimm2019).
The similarities (in morphological characteristics and type of infection) between the metacestodes of V. cuja in ctenomyids from Argentina and those reported by Freeman (Reference Freeman1956) and Niedringhaus et al. (Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021) agree with the idea that V. cuja and the North American species (T. mustelae sensu Freeman (Reference Freeman1956)/Versteria sp. sensu modern authors) are closely related (Bagnato et al., Reference Bagnato, Gilardoni, Martin and Digiani2022). This means that V. cuja could be a new, potentially zoonotic agent in this region of the continent. Indeed, versteriosis due to accidental ingestion by humans of eggs of this species remains hypothetical but should not be disregarded.
Niedringhaus et al. (Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021) also provided a histopathological description of the lesions caused by the metacestodes of Versteria sp. in the liver of O. zibethicus. In their study, post-mortem examination revealed widespread tissue loss and replacement by solid-bodied cestode larvae with minimal adjacent inflammation in many visceral organs.
Similarly to the histological findings reported by Niedringhaus et al. (Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021) for O. zibethicus, the parasitized liver of Ctenomys sp. 1 showed compression of hepatocytes and changes in their arrangement, congested blood vessels and inflammatory infiltrate with predominance of mononuclear lymphocytes. Regarding the histopathological aspect, it is assumed that the presence of the cysticerci not only altered the arrangement and shape of the hepatocytes, but could also have caused a reduction in blood flow and thus, the supply of nutrients, including oxygen, which would have produced degeneration in the hepatocytes adjacent to the atrophied ones. The presence of the capsule as well as the immune cells of the inflammatory infiltrate, corresponding to a parasitic-type infection, would be due to the response of the host's immune system to eliminate the agent. An additional difference with the non-parasitized liver was found in the number of bile ducts. Although in the healthy liver there was one bile duct per portal space, there were approximately 7 bile ducts in the parasitized liver. It is hypothesized that this may be due to the reactivity of the organ by chronicity of the infection (Weiss et al., Reference Weiss, Armstrong and Gagne1997). Hepatitis in the portal space has been associated with biliary hyperplasia in other species (Weiss et al., Reference Weiss, Armstrong and Gagne1997; Lefkowitch, Reference Lefkowitch2005; Fox et al., Reference Fox, Shen, Muthupalani, Rogers, Kirchain and Dewhirst2009; Portas and Taylor, Reference Portas and Taylor2015).
Niedringhaus et al. (Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021) found heavily parasitized lungs with cysticerci cysts, while the lungs of Ctenomys sp. 2 were little parasitized; only 3 cysticerci were observed in the lung parenchyma. The 3 cysts found were observed in the histological sections. Superficially, cysts were seen in the lung as white tumours, and because of this the lung was fixed for histology, but the parasite was not suspected to be the aetiological agent. Despite this variation, there were similar morphological changes such as connective tissue encapsulation and inflammatory response. Other morphological changes such as dilated alveoli and alveoli with eosinophilic content (oedema) were also observed. These 2 histopathological characteristics are probably related to the reduced expansion capacity of the lungs due to the presence of parasitic cysts. In addition to liver, gastrointestinal tract and lungs, Niedringhaus et al. (Reference Niedringhaus, Ganoe, Lovallo, Walter, Yabsley and Brown2021) found other organs infected such as kidneys, ovaries and brain, which was not the case in Ctenomys spp.
This study is the first report of a natural life cycle of a species of Versteria in South America. It integrates taxonomic, morphological, ecological and histopathological aspects of the interaction between a mustelid species, a subterranean rodent and a parasitic tapeworm, with potential transmission to humans.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182023000215.
Data availability
Four of 8 sequences used in the analyses performed here were submitted and are available at GenBank under accession numbers ON980784, OP379709, OP379710 and OP379295.
Acknowledgements
The authors thank Graciela R. Robilotte and José Luis Bagnato for economic support during the study; Mauricio Dromaz, Luis Epele and Baltazar González Chávez for help in the field; Marta Grech for her help with the statistics; Rosa Manzo for facilitating the Hoyer's fluid; Darío Balcazar (Centro de Estudios Parasitológicos y de Vectores -CEPAVE -CCT-CONICET La Plata, Buenos Aires) for molecular analysis (extraction, purification and amplification of ADN); Aldo M. Santo (CCT- CONICET Centro Nacional Patagónico -CENPAT-, Puerto Madryn, Chubut) for histological service. The authors are grateful to the 2 anonymous reviewers who helped to improve the paper. The Dirección Fauna y Flora Silvestre from Ministerio de Agricultura, Ganadería, Industria y Comercio del Chubut for collecting permissions (Resolutions No 098/2018, No 097/2019). F. A., F. B., C. G. B., G. M. M. and M. C. D. are members of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Author contributions
E. B. conceived, designed research, wrote the manuscript and analysed host samples. E. B. and M. C. D. studied the parasites morphologically and molecularly. F. A. and C. G. B. performed the histopathological description. F. B. and G. M. M. captured the rodents. F. B. helped in dissections, taking tissue samples and income of the species to the Mammal Collection. All authors read and approved the manuscript.
Financial support
This work was supported by the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (E. B., PICT N° 2019-00569; M. C. D., PICT No 2019-03535).
Conflict of interest
None.
Ethical standards
Not applicable.