Published online by Cambridge University Press: 24 June 2021
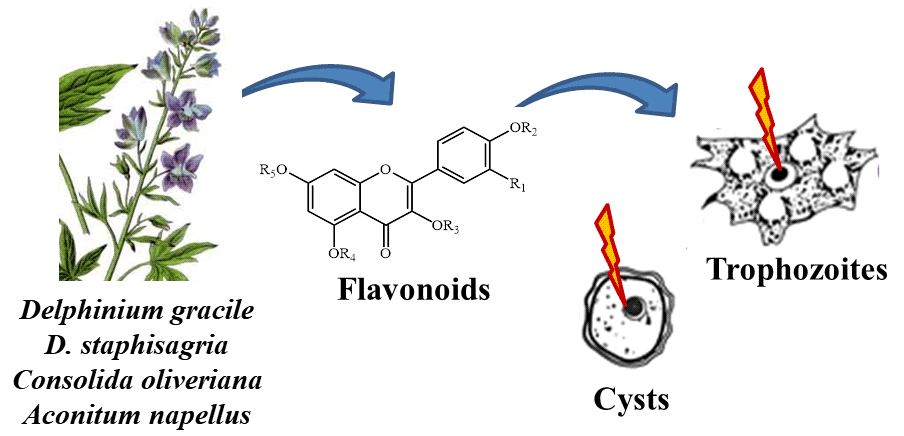
Acanthamoeba spp. are widely distributed in the environment and cause serious infections in humans. Treatment of Acanthamoeba infections is very challenging and not always effective which requires the development of more efficient drugs against Acanthamoeba spp. The purpose of the present study was to test medicinal plants that may be useful in the treatment of Acanthamoeba spp. Here we evaluated the trophozoital and cysticidal activity of 13 flavonoid glycosides isolated from Delphinium gracile, D. staphisagria, Consolida oliveriana and from Aconitum napellus subsp. Lusitanicum against the amoeba Acanthamoeba castellanii. AlamarBlue Assay Reagent® was used to determine the activity against trophozoites of A. castellanii, and cytotoxic using Vero cells. Cysticidal activity was assessed on treated cysts by light microscopy using a Neubauer chamber to quantify cysts and trophozoites. Flavonoids 1, 2, 3 and 4 showed higher trophozoital activity and selectivity indexes than the reference drug chlorhexidine digluconate. In addition, flavonoid 2 showed 100% cysticidal activity at a concentration of 50 μm, lower than those of the reference drug and flavonoid 3 (100 μm). These results suggest that flavonoids 2 and 3 might be used for the development of novel therapeutic approaches against Acanthamoeba infections after satisfactory in vivo evaluations.