Published online by Cambridge University Press: 13 July 2022
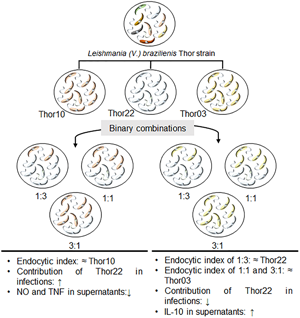
This study focuses on the role of the population structure of Leishmania spp. on the adaptive capacity of the parasite. Herein, we investigate the contribution of subpopulations of the L. (V.) braziliensis Thor strain (Thor03, Thor10 and Thor22) in the profile of murine macrophages infection. Infection assays were performed with binary combinations of these subpopulations at stationary phases. The initial interaction time showed major effects on the combination assays, as demonstrated by the significant increase in the infection rate at 5 h. Based on the endocytic index (EI), Thor10 (EI = 563.6) and Thor03 (EI = 497) showed a higher infection load compared to Thor22 (EI = 227.3). However, the EI decreased in Thor03 after 48 h (EI = 447) and 72 h (EI = 388.3) of infection, and showed changes in the infection level in all Thor10/Thor22 combinations. Assays with CellTrace CFSE-labelled Thor22 promastigotes indicated an increase (~1.5 fold) in infection by this subpopulation in the presence of Thor10 when compared to the infection profile of Thor03/Thor22 combinations in the same proportions. In addition, the potential of these subpopulations, alone or in binary combinations, to modulate the expression of cytokines and nitric oxide (NO) in vitro was investigated. Lower NO and tumour necrosis factor-α production levels were observed for all Thor10/Thor22 combinations at 24 h compared to these subpopulations alone. In contrast, Thor03/Thor22 combination assays increased IL-10 production at this time. Collectively, these results provide in vitro evidence on the potential of L. (V.) braziliensis population structure to play a relevant role in a host infection by this parasite.