Introduction
Angiostrongylus cantonensis, the rat lungworm, is an invasive metastrongylid nematode associated with species of the Rattini, which was recently confirmed in the island of Tenerife (Foronda et al., Reference Foronda, López-González, Miquel, Torres, Segovia, Abreu-Acosta, Casanova, Valladares, Mas-Coma, Bargues and Feliu2010). Currently, the parasite is well established in the humid northern areas of the island (Martín-Carrillo et al., Reference Martín-Carrillo, Feliu, Abreu-Acosta, Izquierdo-Rodriguez, Dorta-Guerra, Miquel, Abreu-Yanes, Martin-Alonso, García-Livia, Quispe-Ricalde, Serra-Cobo, Valladares and Foronda2021). This nematode is known to be a generalist when it comes to paratenic hosts, i.e. its third-stage larvae, which are infective for the definitive, paratenic or accidental hosts, has been described in fish, amphibians and saurians (Wallace and Rosen, Reference Wallace and Rosen1967; Ash, Reference Ash1968; Radomyos et al., Reference Radomyos, Tungtrongchitr, Praewanich, Khewwatchan, Kantangkul, Junlananto and Ayudhya1994). Additionally, several invertebrate species such as planarians or centipedes have been proven to be paratenic or transport hosts of the parasite (Wang et al., Reference Wang, Lu, She, Wen, Mo, Li and Li2018; Chaisiri et al., Reference Chaisiri, Dusitsittipon, Panitvong, Ketboonlue, Nuamtanong, Thaenkham, Morand and Dekumyoy2019). The paratenic hosts have the capacity to accumulate infective larvae and serve as an infection source to avian and mammalian hosts that suffer from neurological disorders like eosinophilic meningitis (Wallace and Rosen, Reference Wallace and Rosen1967; Alicata, Reference Alicata1991; Paredes-Esquivel et al., Reference Paredes-Esquivel, Sola, Delgado-Serra, Puig Riera, Negre, Miranda and Jurado-Rivera2019).
In Tenerife, Rattus rattus and R. norvegicus are definitive hosts of the parasite. The rats have been present in the Canary Islands approximately since the 15th century and occupy practically all habitats where, together with cats, they are the major invasive predators (Nogales et al., Reference Nogales, Rodríguez-Luengo and Marrero2006). The impact of rats in Tenerife's ecosystem not only includes the predation of vertebrates (like lizards), which is probably the biggest negative consequence of their presence, but also to a lesser extent negative effect on invertebrates such as gastropods. Besides the impact of predation, the invasive rodents are an important source of various pathogens (Foronda et al., Reference Foronda, Martin-Alonso, Del Castillo-Figueruelo, Feliu, Gil and Valladares2011; Abreu-Yanes et al., Reference Abreu-Yanes, Martin-Alonso, Martin-Carrillo, Livia, Marrero-Gagliardi, Valladares, Feliu and Foronda2018), including zoonotic nematodes, such as A. cantonensis. Three species of molluscs have been confirmed as intermediate hosts of A. cantonensis in Tenerife: Cornu aspersum, Theba pisana and Plutonia lamarckii (Martin-Alonso et al., Reference Martin-Alonso, Abreu-Yanes, Feliu, Mas-Coma, Bargues, Valladares and Foronda2015).
The terrestrial fauna of Macaronesian archipelago is dominated by reptiles that colonized the islands drifting from the coast of North Africa and further specialized through processes of adaptive radiation (López-Jurado and Mateo, Reference López-Jurado and Mateo1995; Cox et al., Reference Cox, Carranza and Brown2010). Three saurian genera – Gallotia, Chalcides and Tarentola of the families Lacertidae, Scincidae and Gekkonidae – inhabit the islands; however, the terrestrial Gallotia spp. reach fairly highest densities and play an important role in Macaronesian ecosystems (Valido and Nogales, Reference Valido and Nogales1994; Molina-Borja and Bischoff, Reference Molina-Borja, Bischoff, Bischoff and Böhme1998). In Southeast and East Asia, A. cantonensis, saurians are known to be paratenic hosts of A. cantonensis and the consumption of their raw organs or meat by humans has been associated with eosinophilic meningitis outbreaks (Radomyos et al., Reference Radomyos, Tungtrongchitr, Praewanich, Khewwatchan, Kantangkul, Junlananto and Ayudhya1994; Hidelaratchi et al., Reference Hidelaratchi, Riffsy and Wijesekera2005). Similarly, lizards are known to be paratenic hosts in the life cycle of metastrongylids of carnivores (Jeżewski et al., Reference Jeżewski, Buńkowska-Gawlik, Hildebrand, Perec-Matysiak and Laskowski2013). The abundance of lizards in hyperendemic areas of A. cantonensis in Tenerife, together with the diet of these saurians, makes the encounters between G. galloti and infective L3 larvae of A. cantonensis highly probable. The aim of the study was to determine the possible involvement of G. galloti in the life cycle of A. cantonensis in Tenerife.
Materials and methods
Samples collection and the microscopy
Thirty-nine specimens of G. galloti were captured in Tegueste, Tenerife (28°31′32.1″N 16°20′13.9″W) in July 2021. The sampling area was chosen due to the high prevalence of A. cantonensis in rats shown in previous studies (Martín-Carrillo et al., Reference Martín-Carrillo, Feliu, Abreu-Acosta, Izquierdo-Rodriguez, Dorta-Guerra, Miquel, Abreu-Yanes, Martin-Alonso, García-Livia, Quispe-Ricalde, Serra-Cobo, Valladares and Foronda2021) (Fig. 1). Lizards were captured alive with fall traps which were set in the morning and picked up in the afternoon. Once captured, the animals were brought to Instituto Universitario de Enfermedades Tropicales y Salud Pública de Canarias (IUETSPC) where they were anaesthetized using ketamine (Narkamon 100, Bioveta, Czech Republic) and dexmedetomidine (Dexdomitor 0.1, OrionPharma, Czech Republic) intramuscularly, and subsequently euthanized with T61 (MSD, Netherlands) intracardially. During dissection, squashed preparation of a part of liver tissue was examined for the presence of larvae by light microscopy. During microscopical examination, nematode larvae within granulomas were photographed using Leica ICC W camera and five of them were measured using LAS interactive measurement software. Remaining liver tissue was preserved in absolute molecular grade ethanol for the DNA isolation and in 4% formaldehyde. Parts of liver from lizards that presented larvae at microscopy and/or confirmed positive by qPCR were used for histopathological examination. Formalin-preserved liver samples were embedded in paraffin, cut and stained with haematoxylin–eosin. Processed samples were examined using Olympus BX53 microscope.

Fig. 1. Sampling area, Tenerife, Canary Islands.
DNA extraction, PCR and sequencing
Approximately 25 mg of liver tissue and proximal tail muscle tissue were cut into small pieces and used for the DNA extraction with DNEasy Blood&Tissue (Qiagen, Germany) extraction kit with modification optimized for L3 larvae of A. cantonensis, when the pre-lyse phase was extended overnight and 25 μL instead of 20 μL of proteinase K was used.
All 39 liver and 36 tail musculature samples were examined for the presence of A. cantonensis DNA by a species-specific qPCR assay (Sears et al., Reference Sears, Qvarnstrom, Dahlstrom, Snook, Kaluna, Baláž, Feckova, Šlapeta, Modry, Jarvi and Nutman2021) on QuantStudio™ 1 Real-Time PCR System, ThermoFisher at IUETSPC. The assay was performed in a 20 μL reaction using 6.2 μL of PCR water, 10 μL of 2× MasterMix (IDT Prime time gene expression master), 0.2 μL of 10 μ m probe (PrimeTime Eco Probe 5′ 6-FAM/ZEN/3′ IBFQ, /56-FAM/ACA TGA AAC/ZEN/ACC TCA AAT GTG CTT CGA/3IABkFQ/), 0.8 μL of each 10 μ m primer (forward: AAA CTG TTG CTT TCG AAG CTA TG and reverse: GCG CAA ATC TGA CGT TCT TG) and 2 μL of DNA template. Thermocycling (40 cycles) was made with the following cycling conditions: 95°C for 20 s followed by 40°C for 1 s and 60°C for 20 s. As positive controls, DNA from an adult female and a single L3 larva of A. cantonensis extracted by the same method as samples were used. The assay was run in duplicate. Only amplification curves with Ct value under 35 were taken as positive. To obtain the A. cantonensis DNA sequences, conventional PCR was used for amplification of the internal transcribed spacer 2 (ITS-2), partial 18SrDNA and partial COI in qPCR-positive samples of liver; for primers and conditions (see Table 1). Amplicons were sent to Macrogen (Spain and the Netherlands), and resulting sequences were analysed manually using Geneious Prime 2021.0.1 (http://www.geneious.com) and MEGA X and compared with those available in GenBank using BLAST.
Table 1. Primers and conditions used in conventional PCR assays

Results
The microscopic examination showed eight out of 36 samples (22.2%) presenting granulomas with metastrongylid larvae inside (Fig. 2A and B). The length of measured larvae was 573.9 ± 23.7 μm (95%). Histopathological examination of a liver sample (with the presence of A. cantonensis confirmed by qPCR) showed granulomas containing inflammatory cells such as macrophages and lymphocytes, with transversal sections through the nematode larvae (Fig. 2C).
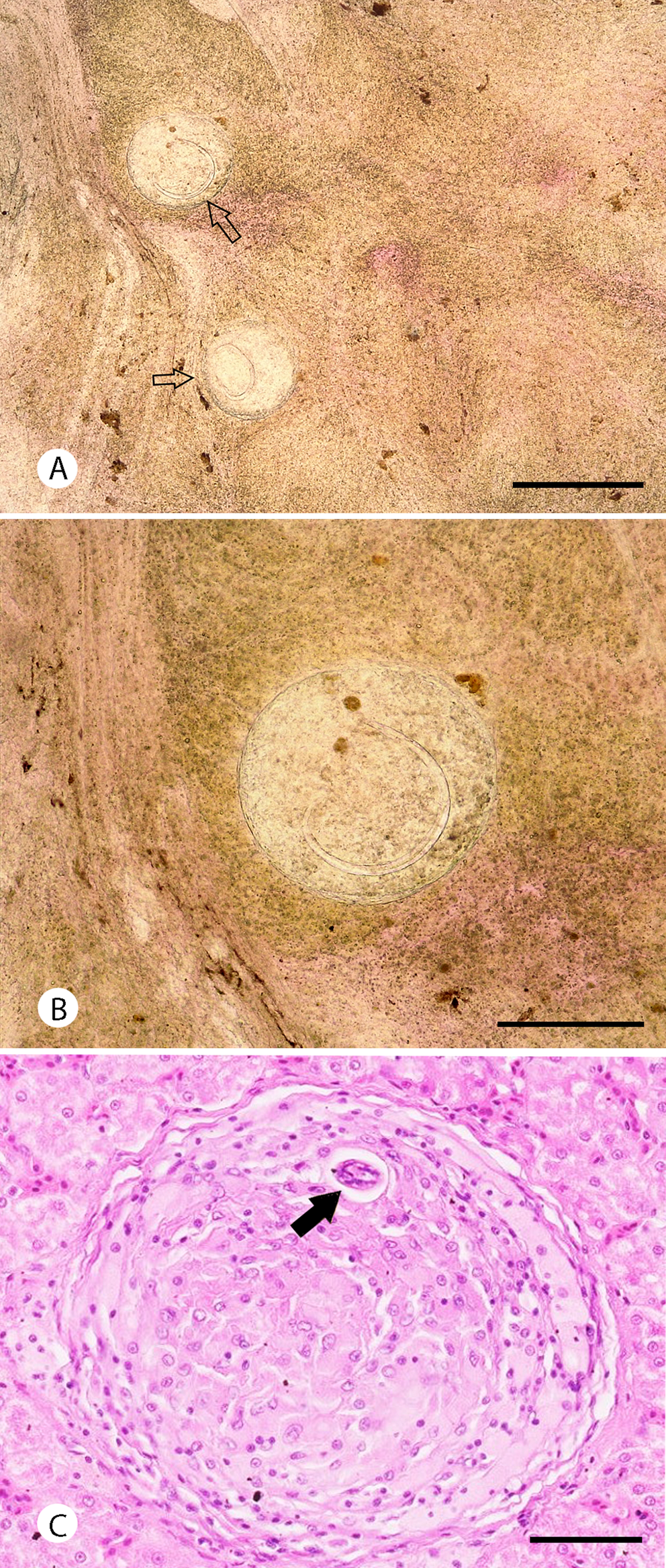
Fig. 2. Metastrongylid larvae in the liver of G. galloti. Squashed preparation of the liver tissues (A, B); granuloma filled with inflammatory cells with visible cross-section through the larva (black arrow). Scale bars: 500 μm (A), 250 μm (B) and 50 μm (C).
qPCR results confirmed the presence of A. cantonensis DNA in liver and tail muscular tissue of G. galloti. Ten out of 39 (25.6%) liver samples and seven out of 36 (19.4%) tail samples tested positive by qPCR with Ct value under 35 cycles. Conventional PCR was only performed in the positive samples by qPCR and confirmed the presence of A. cantonensis DNA in several of these samples as well. Positivity in tail muscles corresponded with DNA detection in the liver in six samples, one sample from the tail showed positive, even though the liver sample from the same individual was negative.
However, not all microscopically positive samples were confirmed positive by qPCR. Five samples where metastrongylid larvae were observed microscopically were negative by A. cantonensis-specific qPCR and, on the contrary, seven samples in which we did not observe larvae microscopically were positive for A. cantonensis DNA when tested by qPCR. Differences between microscopical and molecular analysis are summarized in Table 2.
Table 2. Comparison of results of microscopy of squashed liver samples and molecular methods

Differences in results of microscopy and molecular analysis may indicate not only that qPCR (Sears et al., Reference Sears, Qvarnstrom, Dahlstrom, Snook, Kaluna, Baláž, Feckova, Šlapeta, Modry, Jarvi and Nutman2021) is more sensitive and specific diagnostic method than mere microscopical examination and conventional PCR, but also that possibly other metastrongylid species found G. galloti as a suitable paratenic host.
Note: The positive result of microscopy means just microscopical observation of nematode larvae, not confirmation of A. cantonensis presence.
Two partial COI sequences of length 405 and 635 bp were obtained. When compared to those available in GenBank, these partial sequences were 98.42 and 100% identical to the A. cantonensis lineage TEN.1 from the Tenerife, clustering into the clade 2 with all the invasive lineages, as reviewed by Červená et al. (Reference Červená, Modrý, Fecková, Hrazdilová, Foronda, Martin-Alonso, Lee, Walker, Niebuhr, Malik and Šlapeta2019). Four partial ITS-2 sequences (287–373 bp) and two partial 18S sequences (518 and 619 bp) were obtained, all showing a high level of identity with A. cantonensis (99.6–100%, see Table 2).
Discussion
Angiostrongylus cantonensis is well-known for its complex circulation in food webs (Wallace and Rosen, Reference Wallace and Rosen1967; Alicata, Reference Alicata1991; Paredes-Esquivel et al., Reference Paredes-Esquivel, Sola, Delgado-Serra, Puig Riera, Negre, Miranda and Jurado-Rivera2019). Its life cycle involves rats as definitive hosts, aquatic and terrestrial gastropods as intermediate hosts and a range of poikilotherm paratenic hosts (Wallace and Rosen, Reference Wallace and Rosen1967; Ash, Reference Ash1968; Radomyos et al., Reference Radomyos, Tungtrongchitr, Praewanich, Khewwatchan, Kantangkul, Junlananto and Ayudhya1994). The parasite has been found naturally infecting monitor lizards Varanus bengalensis. These apex predators accumulate high numbers of infective larvae, predominantly present in the liver, much less so in skeletal muscles and the intestine (Radomyos et al., Reference Radomyos, Tungtrongchitr, Praewanich, Khewwatchan, Kantangkul, Junlananto and Ayudhya1994). Infectivity of reptile-derived larvae for a definitive host was confirmed experimentally (Radomyos et al., Reference Radomyos, Tungtrongchitr, Praewanich, Khewwatchan, Kantangkul, Junlananto and Ayudhya1994).
Abundance of G. galloti in Tenerife and their co-occurrence with both invasive rat species makes them ideal paratenic hosts for A. cantonensis. Indeed, we found common presence of metastrongylid larvae in the liver of G. galloti microscopically and confirmed the presence of A. cantonensis by species-specific qPCR and ITS2, COI and 18S sequencing. Considering high sensitivity and specificity of the qPCR assay used (Sears et al., Reference Sears, Qvarnstrom, Dahlstrom, Snook, Kaluna, Baláž, Feckova, Šlapeta, Modry, Jarvi and Nutman2021), the observed partial discrepancy between microscopy and qPCR data suggests the co-occurrence of A. cantonensis larvae together with those of other metastrongylid nematodes. These undetermined larvae might belong to Angiostrongylus vasorum, Aelurostrongylus abstrusus or Crenosoma striatum, which were previously reported from the Canary archipelago (Sánchez Vicente, Reference Sánchez Vicente2013; Segeritz et al., Reference Segeritz, Cardona, Taubert, Hermosilla and Ruiz2021). Further research is needed to clarify the role of endemic lizards in the life cycle of metastrongylids in Macaronesian terrestrial ecosystems and the potential impact of these infections on the fitness of lizards with high ecological value.
Gallotia galloti is a dominant reptile in Tenerife and its altitudinal distribution ranges from sea level until more than 3000 m (Báez, Reference Báez, Pleguezuelos, Márquez and Lizana2002). As a result, the diet of this lizard species varies according to ecosystem type, climate and season; however, plant material (leaves, flowers and fruits) always represents its major part (Valido and Nogales, Reference Valido and Nogales1994, Reference Valido and Nogales2003). Terrestrial gastropods were demonstrated as a minor part of the diet of G. galloti during the colder months (Valido et al., Reference Valido, Nogales and Medina2003).
Gallotia spp. play an important role in the island ecology, not only as seed dispersers, but also as part of the diet of native and alien carnivores in the archipelago which can expose these predators to the infective larvae of A. cantonensis. In birds, G. galloti remnants were identified in 14.2 and 26.1% of samples from Canarian kestrels Falco tinnunculus canariensis and common ravens Corvus corax, respectively (Nogales and Hernández, Reference Nogales and Hernández1994; Molina-Borja and Bischoff, Reference Molina-Borja, Bischoff, Bischoff and Böhme1998; Carrillo et al., Reference Carrillo, González-Dávila and Ruiz2017). Also, the Southern grey shrike Lanius meridionalis feeds on lizards (Padilla et al., Reference Padilla, Nogales and Marrero2007). In the case of mammals, G. galloti is an important part of the diet of feral cats (Felis silvestris catus), being present in 74.2% of total fecal samples analysed in some areas of Tenerife (Nogales et al., Reference Nogales, Abdola, Alonso and Quilis1990; Medina and Nogales, Reference Medina and Nogales2008). Rarely G. galloti has been also reported as a prey for the common frog (Pelophylax perezi), red-backed shrike (Lanius collurio) and the sparrowhawk (Accipiter nisus) (Nogales et al., Reference Nogales, Luis and Alonso1988; Barone and Trujillo, Reference Barone and Trujillo1997; Barone et al., Reference Barone, Hernández and Vizcaíno2006).
The high infection rate of G. galloti with A. cantonensis implies the possibility of its transmission to accidental avian hosts in which it could potentially cause neurological diseases (Monks et al., Reference Monks, Carlisle, Carrigan, Rose, Spratt, Gallagher and Prociv2005; Ma et al., Reference Ma, Dennis, Rose, Spratt and Spielman2013). Since a fatal case of cerebral infection due to A. cantonensis was previously described in a pygmy falcon (Polihierax semitorquatus) implying a gecko as the probable cause of infection (Burns et al., Reference Burns, Bicknese, Qvarnstrom, DeLeon-Carnes, Drew, Gardiner and Rideout2014), it is likely that infection of Canarian kestrels can impact on their fitness in localities with high prevalence of the infection in lizards. It has been suggested that invasive rats may feed on juveniles and eggs of Gallotia spp. (Pleguezuelos et al., Reference Pleguezuelos, Márquez and Lizana2002). However, as there is a lack of data regarding the amount of lizards eaten by rats, the extent in which infected Gallotia contribute to the infection of rats as definitive hosts remains unclear.
Recently described intermediesis, i.e. transmission of metastrongylid L3 larvae between two susceptible hosts at the same trophic level (i.e. gastropods, Colella et al., Reference Colella, Giannelli, Brianti, Ramos, Cantacessi, Dantas-Torres and Otranto2015; Modrý et al., Reference Modrý, Fecková, Putnová, Manalo and Otranto2020), opens a question of transmission of A. cantonensis among lizards by cannibalism. Such a unique mode of transmission has been proven in dihomoxenous apicomplexan protists of Gallotia, such as Sarcocystis gallotiae (Matuschka and Bannert, Reference Matuschka and Bannert1987).
Although the date and way of arrival of A. cantonensis to Tenerife is still uncertain, the high prevalence in rats and its presence among endemic intermediate and paratenic hosts (P. lamarckii, G. galloti) evidence that the parasite is firmly established in local ecosystem (Martin-Alonso et al., Reference Martin-Alonso, Abreu-Yanes, Feliu, Mas-Coma, Bargues, Valladares and Foronda2015; Martín-Carrillo et al., Reference Martín-Carrillo, Feliu, Abreu-Acosta, Izquierdo-Rodriguez, Dorta-Guerra, Miquel, Abreu-Yanes, Martin-Alonso, García-Livia, Quispe-Ricalde, Serra-Cobo, Valladares and Foronda2021). Gallotia galloti is endemic to Tenerife and La Palma; however, its accidental introduction to other islands has been reported (Rodríguez-Domínguez and Ruiz-Caballero, Reference Rodríguez-Domínguez and Ruiz-Caballero1998; Mateo-Miras and Pérez-Mellado, Reference Mateo-Miras and Pérez-Mellado2005). Dispersal of G. galloti outside of its original range of distribution might increase the risk of spread of A. cantonensis to new islands.
Acknowledgements
The authors would like to thank MRIR for his contribution to the lizard capture. Animal trapping and use was approved by the Canary Government in accordance with the Law 42/2007 with the expedient number 2021/29507.
Author contributions
L. Anettová and E. Izquierdo-Rodriguez performed the molecular and microscopical analysis under the guidance and supervision of P. Foronda, V. Baláž and D. Modrý. qPCR analysis was made by L. A. and E. I. R. performed the fieldwork including lizard capturing. L. N. performed histopathological processing and examination. V. B. supervised the qPCR analysis. L. A. and E. I. R. contributed equally.
Financial support
Fieldwork of L. Anettová was funded by Masaryk University's PhD Mobility scholarship. E. Izquierdo-Rodriguez was funded by the scholarship M-ULL (becas M-ULL, convocatoria 2019). The study was also supported by ProID2021010013 [Consejería de Economía, Industria, Comercio y Conocimiento (Gobierno de Canarias) and FEDER CANARIAS 2014-2020], University of La Laguna and the Canary Council of Economy Knowledge and Employment (CEI program). Detection of A. cantonensis in animal tissues was funded by project SEAEUROPEJFS19IN-053 and the Czech Science Foundation grant no. 22-26136S.
Conflict of interest
None.
Ethical standards
The authors declare that all ethical standards and requirements regarding handling and sacrificing vertebrate species in this study were fulfilled.








