Introduction
Small strongyles or cyathostomins are a large group of gastrointestinal parasitic nematodes infecting grazing equids. Cyathostomins encompass more than 50 species able to infect domestic and wild equids (Lichtenfels et al., Reference Lichtenfels, Kharchenko and Dvojnos2008), among which a limited group of 10–12 species emerges as a prevalent and abundant parasitic core consistently found worldwide (Reinemeyer et al., Reference Reinemeyer, Smith, Gabel and Herd1984; Bucknell et al., Reference Bucknell, Gasser and Beveridge1995; Lyons et al., Reference Lyons, Drudge and Tolliver2000; Collobert-Laugier et al., Reference Collobert-Laugier, Hoste, Sevin and Dorchies2002; Osterman Lind et al., Reference Osterman Lind, Eysker, Nilsson, Uggla and Höglund2003, Reference Osterman Lind, Kuzmina, Uggla, Waller and Höglund2007; Kuzmina et al., Reference Kuzmina, Kharchenko, Starovir and Dvojnos2005, Reference Kuzmina, Lyons, Tolliver, Dzeverin and Kharchenko2012). The main clinical effect of cyathostominosis is weight loss, and the massive simultaneous emergence of larval stages encysted in the colonic mucosa, can be fatal for horses especially for young animals (Love and Duncan, Reference Love and Duncan1992; Love et al., Reference Love, Murphy and Mellor1999; Peregrine et al., Reference Peregrine, Molento, Kaplan and Nielsen2014). This larval cyathostominosis syndrome, characterized by protein-losing enteropathy, diarrhoea and colic (Giles et al., Reference Giles, Urquhart and Longstaffe1985), remains the leading cause of parasite-mediated death of young horses in some areas like France (Sallé et al., Reference Sallé, Guillot, Tapprest, Foucher, Sevin and Laugier2020). Cyathostominosis control has long been achieved mainly through the use of chemical anthelmintics (Love, Reference Love2003; Peregrine et al., Reference Peregrine, Molento, Kaplan and Nielsen2014), but this has promoted the selection of isolates resistant to all anthelmintics used in equine medicine (Kaplan, Reference Kaplan2002, Reference Kaplan2004; Traversa et al., Reference Traversa, Castagna, von Samson-Himmelstjerna, Meloni, Bartolini, Geurden, Pearce, Woringer, Besognet, Milillo and D'Espois2012; Matthews, Reference Matthews2014). Macrocyclic lactones, including ivermectin (IVM) and moxidectin, remain the most effective drug class against small strongylids (Peregrine et al., Reference Peregrine, Molento, Kaplan and Nielsen2014; Sallé et al., Reference Sallé, Cortet, Bois, Dubès, Guyot-Sionest, Larrieu, Landrin, Majorel, Wittreck, Woringer, Couroucé, Guillot, Jacquiet, Guégnard, Blanchard and Leblond2017; Nielsen et al., Reference Nielsen, Banahan and Kaplan2020). They also remain the most widely used anthelmintic drugs in equine operations in the USA (Becher et al., Reference Becher, van Doorn, Pfister, Kaplan, Reist and Nielsen2018; Nielsen et al., Reference Nielsen, Banahan and Kaplan2020) or in the UK, where IVM and moxidectin are included in drenching programmes in more than a third of cases (Stratford et al., Reference Stratford, Lester, Morgan, Pickles, Relf, McGorum and Matthews2014; Tzelos et al., Reference Tzelos, Morgan, Easton, Hodgkinson and Matthews2019). However, reduced IVM efficacy has been evidenced (Nielsen et al., Reference Nielsen, Banahan and Kaplan2020), including a reduced egg reappearance period (ERP) (Lyons et al., Reference Lyons, Tolliver, Collins, Ionita, Kuzmina and Rossano2011a, Reference Lyons, Tolliver and Collins2011b; Nielsen et al., Reference Nielsen, Banahan and Kaplan2020). In addition, high concentrations of these molecules are found in the feces of treated animals, with major detrimental effects on coprophagous organisms (Lumaret et al., Reference Lumaret, Errouissi, Floate, Römbke and Wardhaugh2012; Verdú et al., Reference Verdú, Lobo, Sánchez-Piñero, Gallego, Numa, Lumaret, Cortez, Ortiz, Tonelli, García-Teba, Rey, Rodríguez and Durán2018).
This context warrants alternative strategies to the use of chemical anthelmintics, and the prospect of using bioactive plant compounds as nutraceuticals for the control of gastrointestinal parasites in livestock has been an active research field (Paolini et al., Reference Paolini, Dorchies and Hoste2003; Heckendorn et al., Reference Heckendorn, Häring, Maurer, Senn and Hertzberg2007; Rochfort et al., Reference Rochfort, Parker and Dunshea2008; Manolaraki et al., Reference Manolaraki, Sotiraki, Stefanakis, Skampardonis, Volanis and Hoste2010; Sandoval-Castro et al., Reference Sandoval-Castro, Torres-Acosta, Hoste, Salem and Chan-Pérez2012; Hoste et al., Reference Hoste, Torres-Acosta, Sandoval-Castro, Mueller-Harvey, Sotiraki, Louvandini, Thamsborg and Terrill2015). Tannin-rich plants are studied for their anthelmintic activity and their use as bioactive forage (Hoste et al., Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin2006, Reference Hoste, Martinez-Ortiz-De-Montellano, Manolaraki, Brunet, Ojeda-Robertos, Fourquaux, Torres-Acosta and Sandoval-Castro2012; Gaudin et al., Reference Gaudin, Simon, Quijada, Schelcher, Sutra, Lespine and Hoste2016; Peña-Espinoza et al., Reference Peña-Espinoza, Valente, Thamsborg, Simonsen, Boas, Enemark, López-Muñoz and Williams2018).
Among tannin-rich plants, sainfoin (Onobrychis viciifolia) is often used as a model fodder (Hoste et al., Reference Hoste, Martinez-Ortiz-De-Montellano, Manolaraki, Brunet, Ojeda-Robertos, Fourquaux, Torres-Acosta and Sandoval-Castro2012). Some in vitro tests on ruminant gastrointestinal nematodes have shown that sainfoin and the tannins reduce larval migration (Paolini et al., Reference Paolini, Fouraste and Hoste2004; Manolaraki et al., Reference Manolaraki, Sotiraki, Stefanakis, Skampardonis, Volanis and Hoste2010). In vivo studies have reported a decrease in fecal egg counts (FECs), associated with a reduction in worm fertility or counts in goats or sheep fed with sainfoin (Paolini et al., Reference Paolini, Dorchies and Hoste2003, Reference Paolini, De La Farge, Prevot, Dorchies and Hoste2005; Heckendorn et al., Reference Heckendorn, Häring, Maurer, Zinsstag, Langhans and Hertzberg2006; Manolaraki et al., Reference Manolaraki, Sotiraki, Stefanakis, Skampardonis, Volanis and Hoste2010; Gaudin et al., Reference Gaudin, Simon, Quijada, Schelcher, Sutra, Lespine and Hoste2016). In horses, a previous study (Collas et al., Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2018) evaluated the anthelmintic activity of dehydrated sainfoin pellets on equine strongyles. While strongyle FEC did not differ between horses fed with sainfoin compared to the control group, an in vitro approach using sainfoin pellet water solutions revealed a decrease in egg hatching and larval development (Collas et al., Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2018). However, it remains unclear whether the latter effects can be reproduced in vivo and if the putative effects of sainfoin extracts vary across various cyathostomin species. The recent development of an ITS-2-based metabarcoding approach has allowed the simultaneous identification and quantification of strongyle abundances in domestic horses (Mitchell et al., Reference Mitchell, O'Sullivan, Pinloche, Wilkinson, Morphew and McEwan2019; Poissant et al., Reference Poissant, Gavriliuc, Bellaw, Redman, Avramenko, Robinson, Workentine, Shury, Jenkins, McLoughlin, Nielsen and Gilleard2021) or wild equids (Tombak et al., Reference Tombak, Hansen, Kinsella, Pansu, Pringle and Rubenstein2021). This approach has been used to characterize the response to anthelmintic treatment of trichostrongylids in ruminants (Queiroz et al., Reference Queiroz, Levy, Avramenko, Redman, Kearns, Swain, Silas, Uehlinger and Gilleard2020; Halvarsson and Höglund, Reference Halvarsson and Höglund2021) or the monitoring of drug resistance in these species (Queiroz et al., Reference Queiroz, Levy, Avramenko, Redman, Kearns, Swain, Silas, Uehlinger and Gilleard2020). It has never been applied yet to study a plant extract effect on any gut parasite community.
In addition to their anthelmintic activity, tannin-rich plants contain flavonoids. These molecules modulate ATP binding cassette (ABC) transporters (Morris and Zhang, Reference Morris and Zhang2006), thereby affecting the in vitro and in vivo pharmacodynamics and activity of macrocyclic lactones (Lespine et al., Reference Lespine, Alvinerie, Vercruysse, Prichard and Geldhof2008). For instance, quercetin significantly increases exposure to moxidectin in lambs (Dupuy et al., Reference Dupuy, Larrieu, Sutra, Lespine and Alvinerie2003). The tannin-rich plant redberry juniper (Juniperus pinchotii) used in combination with oral IVM treatment has also increased the treatment efficacy against Haemonchus contortus in lambs (Whitney et al., Reference Whitney, Wildeus and Zajac2013). However, an in vivo trial in sheep reported a significant decrease in IVM efficacy in sainfoin-fed animals due to a concomitant reduction in plasma IVM concentrations (Gaudin et al., Reference Gaudin, Simon, Quijada, Schelcher, Sutra, Lespine and Hoste2016). Such interaction remains uncharacterized in horses to date.
To bridge this knowledge gap, the current study aimed (i) to evaluate the in vivo effect of dehydrated sainfoin pellets on cyathostomins (fecal egg excretion and larval development), (ii) to quantify any alterations of the cyathostomin larval community structure using a nemabiome approach, and (iii) to establish how the sainfoin diet may affect the efficacy of an oral IVM treatment in horses.
Materials and methods
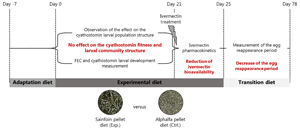
The in vivo experiment was conducted from September 8th to December 15th 2020, at the French Horse and Riding Institute experimental farm (IFCE) in Chamberet, France. The experimental procedure received approval from the French Ministry of Research under protocol number APAFIS#26140-2020062216271790v2. The experimental design is summarized in Fig. 1.
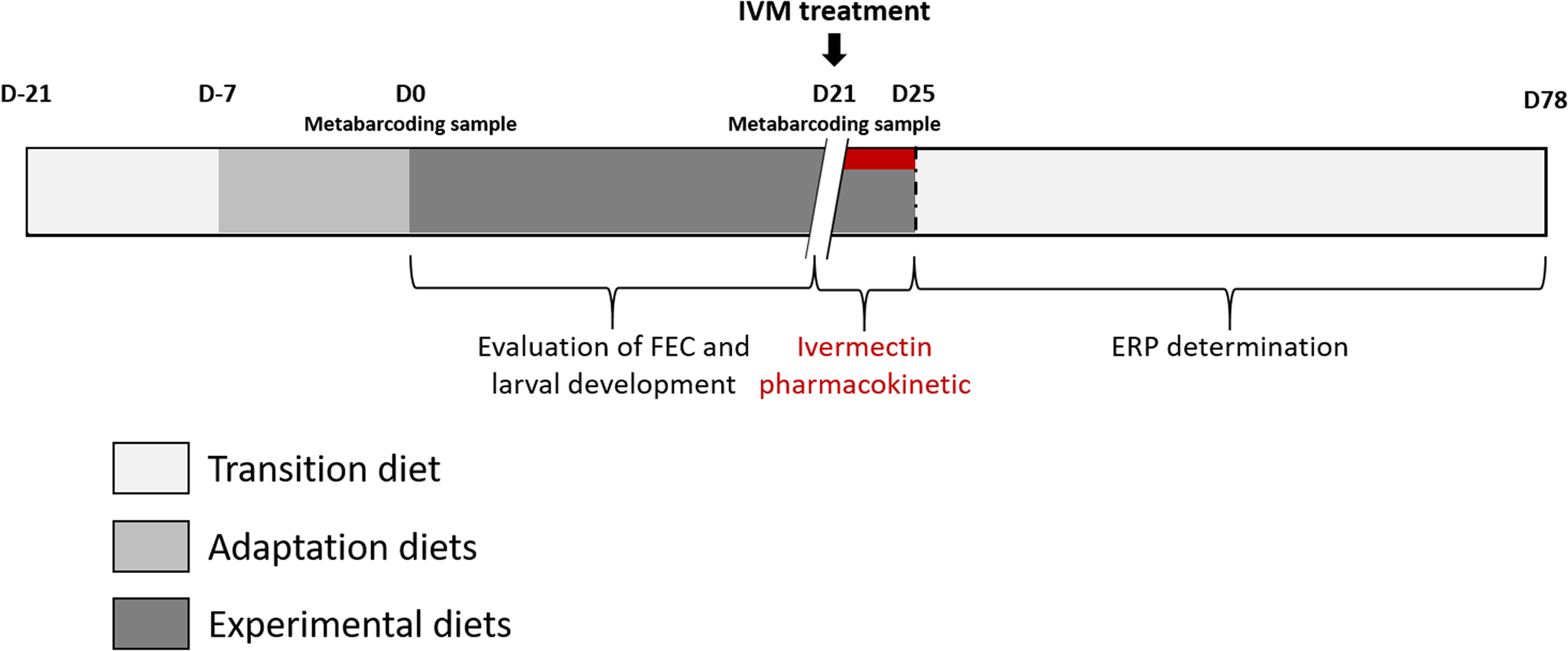
Fig. 1. Experimental design. The figure depicts the time points and sampling done in this experiment.
Animal condition
Naturally infected saddle horses (Anglo-Arab breed, 2 years old) left undrenched for 137 days (last anthelmintic administered on 24 April 2020; 200 μg IVM kg−1 body weight (BW) and 1 mg praziquantel kg−1 BW; EQVALAN® DUO, France) were allocated into 2 groups of 10 horses. Horses were mainly infected by small strongylids (>95%), as revealed by larval culture (Collas et al., Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2018) and the herein reported nemabiome approach which did not disclose the presence of Strongylus spp. Both groups were balanced for sex (8 females and 2 geldings in each group), FEC data before housing (September 8th 2020; sainfoin group FEC = 1875 ± 1253 eggs g−1 (EPG); control group FEC = 1940 ± 1064 EPG, mean ± s.d.), and for their BW measured on September 15th (average BW of 454.8 ± 28.1 kg and 457.6 ± 25.6 kg in the sainfoin and control diet groups, respectively). From September 8th to 21th (day-21 to day-8; see Fig. 1), all horses were housed in a single stall and collectively fed with a transition diet composed of 77% grassland hay and 23% of concentrate (made of 61.5% of barley, 35% of soya bean meal, 3.5% of minerals and vitamins), designed to meet their energy (6.0 UFC day−1) (UFC; horse feed unit) and protein requirements (318 g MADC day−1) (MADC; horse digestible crude protein), as previously defined for this breed (INRA, 2015).
Experimental diet
Horses were adapted (adaptation period) to their experimental diet for a week – from day-7 to day 0 (Fig. 1), i.e. the horses were fed individually with a decreasing proportion of the transition diet and an increasing proportion of the experimental diet. They subsequently received their experimental diet for the 3 following weeks (day 0–21, Fig. 1). The horses were housed in individual stalls during these 2 periods (from day-7 to day 21; see Fig. 1). This duration was in line with successful experiments in ruminants (Paolini et al., Reference Paolini, De La Farge, Prevot, Dorchies and Hoste2005; Heckendorn et al., Reference Heckendorn, Häring, Maurer, Zinsstag, Langhans and Hertzberg2006) and with practical implementation in the field. The sainfoin diet contained 2.3% dry matter (DM) of condensed tannins as determined by an acetone–butanol–HCl assay (Grabber et al., Reference Grabber, Zeller and Mueller-Harvey2013); it included 70% DM Equifolia dehydrated sainfoin pellets (Onobrychis viciifolia; provided by Multifolia, Viapres-le-Petit, France) and 30% grassland hay (on DM bases). We worked with a production chain for dehydrated sainfoin pellets, which ensured appropriate agronomic conditions for the plant cultivation, a good conservation of sainfoin characteristics over time and the standardization and characterization of the batches before use. The control diet consisted of 60% DM of alfalfa (Medicago sativa) pellets and 40% DM of grassland hay (Table 1; Supplementary Table S1). Proportions of diet components were chosen to maximize the amount of condensed tannins in the sainfoin diet (while taking care not to cause digestive problems) and to ensure each diet covered horse energy and protein requirements. The energy requirements (UFC) were covered at 108% ± 1.3 and 103% ± 1.5 on average for the sainfoin and control groups, respectively. This difference was statistically significant between the 2 groups (P < 10−4), but our previous observations have shown that energy requirements were not affecting the outcome of the comparison (Collas et al., Reference Collas, Fleurance, Cabaret, Martin-Rosset, Wimel, Cortet and Dumont2014). The protein requirements (MADC) were covered at 250% ± 3.7 and 252% ± 5.5 on average for the sainfoin and control groups, respectively (P = 0.13). These values were safe for the duration of the treatment and showed no significant correlation with FEC or larval development rate (P = 0.45 and P = 0.89, respectively), in good agreement with past experimental findings (Collas et al., Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2018). Individual requirements were estimated based on INRA (2015) tables for UFC and MADC as
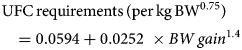
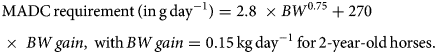
Table 1. Chemical composition and nutritive value of foodstuffs offered to the 2 groups (sainfoin diet, control diet) of horses during the experimental perioda
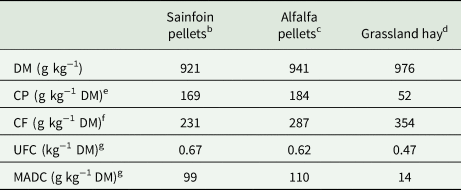
DM, dry matter; CP, crude protein; CF, crude fibre; UFC, horse feed unit; MADC, digestible protein.
a Analyses were performed by UpScience, Saint-Nolff, France, on 4 samples of each foodstuff collected all along the experimental period.
b,dIn sainfoin diet.
c,dIn control diet.
e Dumas method.
f Weende method.
g From INRA (INRA, 2015) equations.
Horses received half of their diet at 8:00 am and the other half at 4:00 pm. Quantities offered were adjusted every Tuesday based on BW changes and the DM content of diet components. If an individual horse lost weight, its BW measured the week before was used to determine its requirements. Individual refusals were weighed every morning, and they never exceeded 5% DM of the offered diet for more than 3 consecutive days, thereby warranting any particular adaptation.
Fecal sample analysis
Fecal samples were collected individually from the rectum of each horse every Monday of the experimental period (day 0, day 7, day 14 and day 21). Samples were stored at +4 °C and shipped to the INRAE Centre Val de Loire facilities (Nouzilly, France) for further processing. Individual FEC data were determined using a modified McMaster technique (Raynaud et al., Reference Raynaud, William and Brunault1970) based on 5 g of fecal matter diluted with a dilution factor of 5. Eggs were then counted using optical microscopy (×150 magnification), with a minimum detection limit of 50 EPG. To evaluate the effect of the diet on larval development, the remaining fecal matter (40–90 g) was incubated individually for each horse for 12 days at +25 °C and 60% relative humidity during 2 weeks as suggested by Roberts and O'Sullivan (Reference Roberts and O'Sullivan1950). The correlation between the quantity of fecal matter cultured and the larval development was not significant (Spearman's ρ = 0.08, P = 0.4). Infective third-stage larvae (L3) of cyathostomins were then collected using a Baermann apparatus after 24 and 48 h of sedimentation, and pooled together for each horse and time point. To count larval concentration, 30 drops of 5 μL were taken from a homogenized larval suspension (using a bar magnet in a glass beaker) and inspected using optical microscopy. The average number of larvae across the 30 drops was related to the total volume of larval suspension collected after Baermann. Following Collas et al. (Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2018), the larval development rate was then derived as:
Gastrointestinal nemabiome
To identify the putative effects of sainfoin on the gastrointestinal nemabiome, a metabarcoding approach was applied to cyathostomin larval populations (using pools of 20 000 L3) harvested from fecal samples collected on day 0 and day 21 of the experiment. Larvae were incubated with 10 μL of a 20 mg mL−1 proteinase K (Qiagen) solution at 56 °C for 3 h; DNA was subsequently extracted using a phenol–chloroform protocol (Sambrook and Russell, Reference Sambrook and Russell2006) and eluted in a 30 μL TE (Tris-EDTA) buffer solution. For metabarcoding, the ITS-2 gene region was PCR amplified using the NC1 (5′-ACGTCTGGTTCAGGGTTGTT-3′) and NC2 (5′-TTAGTTTCTTTTCCTCCGCT-3′) primers (Gasser et al., Reference Gasser, Chilton, Hoste and Beveridge1993). Between 1 and 3 random bases were added to the 5′ primer end to increase sequence complexity. Moreover, a 2-bp linker and an Illumina 28-bp overhang were added for the forward and reverse sequences, respectively, for subsequent ligation with Illumina adapters. PCR was run for 3 min at 95 °C for the first denaturation, then 30 cycles starting with 15 s at 98 °C, then 60 °C for 15 s and 72 °C for 15 s, followed by a final extension of 72 °C for 2 min. PCR products were then loaded on a 1% agarose gel to validate the presence of an amplicon product. After this step, 31 out of the 40 samples from 16 horses remained for library preparation. Because MiSeq enables paired 250-bp reads, the ends of each read are overlapped and can be stitched together to generate extremely high-quality, full-length reads of the entire region in a single run. Single multiplexing was performed using a homemade 6 bp index, which was added to reverse primer during a second PCR with 12 cycles using forward primer (AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGAC) and reverse primer (CAAGCAGAAGACGGCATACGAGAT-index-GTGACTGGAGTTCAGACGTGT). The resulting PCR products were purified and loaded onto the Illumina MiSeq cartridge according to the manufacturer's instructions. Libraries were further processed for a single run of MiSeq sequencing using 500 cycle reagent kit v3 (2 × 250 output; Illumina, USA), the raw data of which are registered under SRA bioproject number PRJNA840924. The quality of the run was checked internally using PhiX, and then each paired-end sequence was assigned to its sample with the help of the previously integrated index.
To assess the predictive ability of the nemabiome approach and determine the best set of parameters for data processing, five mock communities of known species composition were built from worms collected and morphologically identified in Ukraine (T. A. Kuzmina, personal communication) or Poland (M. Basiaga, personal communication). Two mock communities of 5 species (a single male worm each), including Cylicocyclus nassatus, Cylicocyclus insigne, Cyathostomum catinatum, Cyathostomum pateratum and Coronocyclus labiatus were considered using either raw or equimolar DNA concentrations. In addition, two mock communities comprising C. pateratum and C. catinatum (equivalent proportion or in a 1:4 ratio) were also considered to establish the ability of the ITS-2 sequence to delineate between these 2 phylogenetically close species (Hung et al., Reference Hung, Chilton, Beveridge and Gasser2000). Raw reads were processed with Cutadapt v.1.14 (Martin, Reference Martin2011) to remove bad quality bases at the 3′ end of reads (-q 15), trim primer sequences, remove sequences with evidence of indels (--no-indels) or that showing no trace of primer sequence (--discard-untrimmed). Quality filtered ITS-2 amplicon sequences were subsequently handled with the dada2 algorithm (Callahan et al., Reference Callahan, McMurdie, Rosen, Han, Johnson and Holmes2016). A set of different parameters was tested to measure the total number of amplicon sequence variants (ASVs), the rate of taxonomic assignment, and the proportion of false-positive and false-negative detection in mock community samples. First, the maximum error rate (--max-ee) was set to 1 for both reads or 2 and 5 for forward and reverse reads, respectively. The read truncation parameter was either 200 bp or 217 bp, corresponding to the minimal average read length measured across all samples as determined with FastQC v.0.11.7 (Andrews, Reference Andrews2010). The BAND_SIZE parameter effect (that penalizes the number of insertions permitted when aligning 2 sequences) was tested using either the default or the ITS-2 recommended values (16 and 32, respectively), or disabling banding (BAND_SIZE = −1) to perform a full Needleman–Wunsch alignment as described elsewhere (Poissant et al., Reference Poissant, Gavriliuc, Bellaw, Redman, Avramenko, Robinson, Workentine, Shury, Jenkins, McLoughlin, Nielsen and Gilleard2021). Denoising was performed using the pseudo-pool option, and chimaera removal was performed under the default consensus mode. Taxonomic classification was subsequently done using the curated ITS-2 database (https://www.nemabiome.ca/its2-database.html), last accessed on 2 February 2022 (Workentine et al., Reference Workentine, Chen, Zhu, Gavriliuc, Shaw, de Rijke, Redman, Avramenko, Wit, Poissant and Gilleard2020). Two taxonomic assignments were considered and compared, considering mock communities as a ground truth. First, the IdTaxa (Murali et al., Reference Murali, Bhargava and Wright2018) function implemented in the DECIPHER package v2.18.1 (Wright, Reference Wright2016) was applied with 100 bootstraps and a threshold of 50%. Second, the dada2 assignTaxonomy (Callahan et al., Reference Callahan, McMurdie, Rosen, Han, Johnson and Holmes2016) function was implemented as already described (Poissant et al., Reference Poissant, Gavriliuc, Bellaw, Redman, Avramenko, Robinson, Workentine, Shury, Jenkins, McLoughlin, Nielsen and Gilleard2021), i.e. setting tryRC = TRUE, minBoot = 0, outputBootstraps = TRUE and retaining ASVs with 50% bootstrap support. In both cases, the number of false-positive and false-negative counts were determined.
Count tables data were further analysed in R v. 4.1 (R Team, 2020) using the phyloseq (McMurdie and Holmes, Reference McMurdie and Holmes2013) and vegan packages (Oksanen et al., Reference Oksanen, Blanchet, Kindt, Legendre, Minchin, O'Hara, Simpson, Solymos, Stevens and Wagner2015). Rare ASVs (<50 counts in total) were considered contaminants and removed. Samples with less than 50 read counts (negative water control; n = 1 at day 21) were also discarded.
Egg reappearance period
At the end of the sainfoin-fed diet (day 21), all horses were treated with oral IVM (Eqvalan, 200 μg kg−1 BW, Boehringer Ingelheim, Lyon, France) and were still fed with their respective experimental diets for 4 days. In addition, the ERP was determined from measurements of individual FECR data on 5 occasions over a 42-day window (on day 36, day 50, day 63, day 71 and day 78). The ERP was defined by WAAVP (Nielsen et al., Reference Nielsen, von Samson-Himmelstjerna, Kuzmina, van Doorn, Meana, Rehbein, Elliott and Reinemeyer2022) as the time when the upper confidence interval for the mean fecal egg count reduction (FECR) fell below the mean of FECR determined 2 weeks post-treatment minus 10%. FECR data were calculated according to the WAAVP guidelines (Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992, Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006) as
where FECd21 – FEC data on day 21, FEC i stands for the FEC measured at each timepoint i between day 36 and day 78.
Ivermectin dosage in plasma and pharmacokinetics parameters
To quantify horse exposure to IVM, blood samples (9 mL) were taken from the jugular vein of each horse in heparinized tubes at 0, 1, 2, 24, 48, 72 and 96 h after IVM treatment. Blood samples were kept at +4 °C before centrifugation (1500 g for 30 min), and the plasma was frozen at −20 °C until further shipment and processing. Plasmatic IVM concentration was determined by high-performance liquid chromatography (HPLC) with fluorescence detection according to previously described and validated methods (Alvinerie et al., Reference Alvinerie, Sutra, Galtier, Lifschitz, Virkel, Sallovitz and Lanusse1999). Data were analysed using a non-compartmental approach with version 4.2 of the Kinetica Tm computer program (innaPhase, Philadelphia, USA). The partial area under the plasma concentration–time curve (AUC) was calculated from 1 to 96 h by the linear trapezoidal rule. C max, maximal concentration was then determined. Data were expressed as geometric mean ± standard error of the mean.
Statistical analysis
To test for the effect of the diet on FEC and larval development rate, a generalized estimating equation model was implemented with the geeglm function of the geepack v.1.3-1 package (Højsgaard et al., Reference Højsgaard, Halekoh and Yan2006).
To test for differences in alpha diversity, a t-test was performed between the Shannon index values estimated for the sainfoin and control group on day 0 and day 21. To test for a differential temporal trend in species abundance between both groups during the experimental diet, species counts were regressed upon the experimental group and the day effects and setting horse as a random effect with the nlme package v.3.1-155. This analysis was restricted to 12 horses with data on both days (n = 8 in the sainfoin group, n = 4 in the control group) and to the most abundant cyathostomin species (overall abundance of 10 000 counts, including C. nassatus, Cylicocyclus ashworthi, Cylicostephanus minutus and C. longibursatus). Species counts were 4th-root transformed to better fit normality (Shapiro–Wilk test = 0.56 and 0.96 before and after transformation, respectively).
FEC measured after IVM treatment was modelled with a linear mixed-effects model using the lme function of the nlme package (Pinheiro et al., Reference Pinheiro, Bates, DebRoy and Sarkar2013), fitting diet, time and diet × time interaction terms as fixed effects and horse as a random effect. The FECR was measured according to the WAAVP guidelines (Coles et al., Reference Coles, Bauer, Borgsteede, Geerts, Klei, Taylor and Waller1992, Reference Coles, Jackson, Pomroy, Prichard, von Samson-Himmelstjerna, Silvestre, Taylor and Vercruysse2006) with Bayesian hierarchical models using the fecrtCI function on the eggCounts package v.3.2-3 (Wang et al., Reference Wang, Paul, Isler and Furrer2022).
The average IVM concentration and C max for the sainfoin group were compared to the control group at every time point (+1, +2, +24, +48, +72 and +96 h). Data were analysed with a Mann–Whitney U test, and the P values were corrected for multiple tests using a Bonferroni procedure as implemented in the wilcox_test function of rstatix package v.0.6.0 (Kassambara, Reference Kassambara2021). The pharmacokinetics (PK) parameters (AUC) for each group were compared with an unpaired t-test, and the P values were corrected by Bonferroni, as explained above.
Results
Sainfoin effect on FEC excretion
When horses received their experimental diet (day 0 to day 21), individual cyathostomin FECs significantly decreased by 35% from 1165 ± 545 EPG to 765 ± 396 EPG on average (P = 0.017, Fig. 2A). However, no significant difference between the sainfoin and the control group was found at any considered time point (P = 0.90).
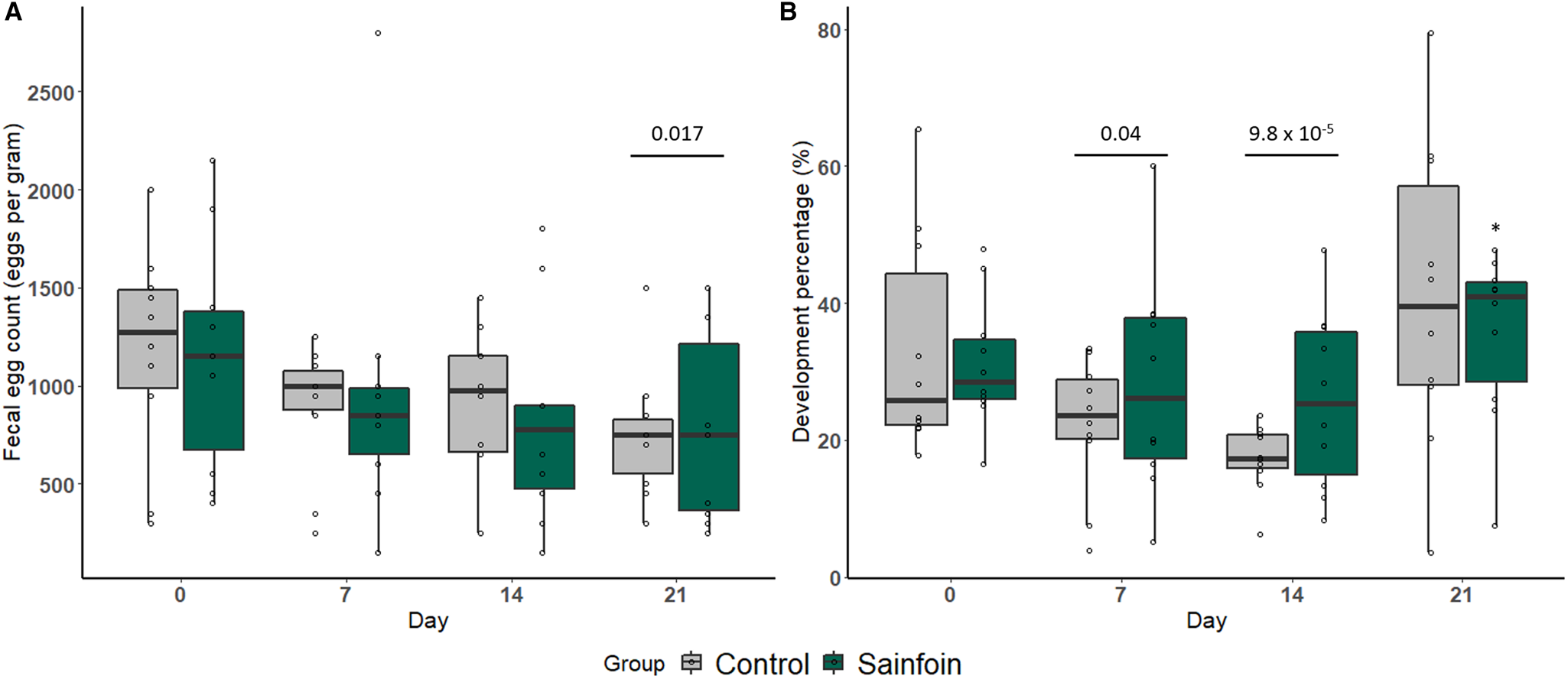
Fig. 2. Arithmetic average of FEC (A) and larval development rate (B) measured over the experimental period. Weekly arithmetic average FEC (A) or larval development rate (B) measured throughout the experimental period in horses receiving the control (light grey) or sainfoin (dark green) diet. The average cyathostomin FEC on day 21 (represented by the bar) was significantly different from the average FEC on day 0 (P = 0.017) (A). *Statistically significant difference in larval development rate from day 14 to day 21 between the sainfoin and control groups (P = 0.02). The bars indicate a significant difference in the mean larval development at day 7 (P = 0.04) and day 14 (P = 9.8 × 10−5), both compared to day 0 (B).
Sainfoin effect on larval development rate
The average larval development rate (Fig. 2B) decreased between day 0 and day 14 of the experiment from an average development of 32.3 ± 12.8% at day 0 to 25.2 ± 13.3% at day 7 (P = 0.04) and 21.5 ± 10.4% at day 14 (P = 9.8 × 10−5). This decrease was similar between the 2 diets (P = 0.27 and 0.06 on day 7 and day 14, respectively). However, the subsequent increase in larval development rate from day 14 to day 21 was significantly lower in the sainfoin-fed group (P = 0.02, Fig. 2B).
Sainfoin effect on cyathostomin larval community structure
Among the combinations of parameters tested, a BAND_SIZE of −1 combined with a truncation length of 217 bp and maximal error rates of 2 and 5 for the forward and reverse reads were optimal. This combination yielded the highest fraction of assigned amplicon sequence variants without false-positive calls in the mock parasite community (83.6% assigned ASVs out of the 110 ASVs detected in total; Supplementary Table S2). Using this set of parameters and after filtering, 31 samples were left for analysis with an average of 6768 reads per sample (ranging between 2269 and 25 434 reads) that defined 110 ASVs. Out of these, 15 were considered contaminants (<50 occurrences), 75 ASVs were assigned to 13 identified cyathostomin species and 6 remained undetermined (amounting to 0.6% of total counts). Members of the Cyathostomum and Cylicocyclus genera accounted for the highest misassignment rate at the species level (5.78 and 2.75% of total counts, respectively), while unassigned Cylicostephanus spp. represented 0.09% of total counts. To this respect, taxonomic assignment with the Idtaxa function performed better than the assignTaxonomy function that consistently introduced false-positive identification of C. longibursatus instead of Cyathostomum species. In line with this higher misassignation rate, the inferred proportions of Cyathostomum species in respective mock communities departed from the expected ratio, with a bias towards C. catinatum relative to C. pateratum (62.3 and 92.7% in lieu of 50 and 75% expected, Supplementary Figure S1).
On day 0, Cylicostephanus minutus was the most abundant species in the gastrointestinal nemabiome (13.83% of overall counts, Fig. 3), followed by C. ashworthi and C. nassatus (5.47 and 5.06% of total counts on that day, respectively, Fig. 3). At the community level, we could not evidence any differences in alpha diversity between larval communities of the sainfoin and control groups, neither at the beginning [average Shannon index difference of 0.18, 95% c. i. = (−0.71; 0.35), P = 0.47] nor at the end of the experimental period [average Shannon index difference of 0.16, 95% c. i. = (−0.72; 0.41), P = 0.53]. Similarly, PERMANOVA analyses did not evidence any difference between the larval community structures of the 2 groups on day 0 (P = 0.47 and P = 0.2 with the Bray-Curtis and Jaccard dissimilarity index, respectively) or day 21 (P = 0.24 and P = 0.55 with the Bray-Curtis and Jaccard dissimilarity index, respectively) of the experiment.
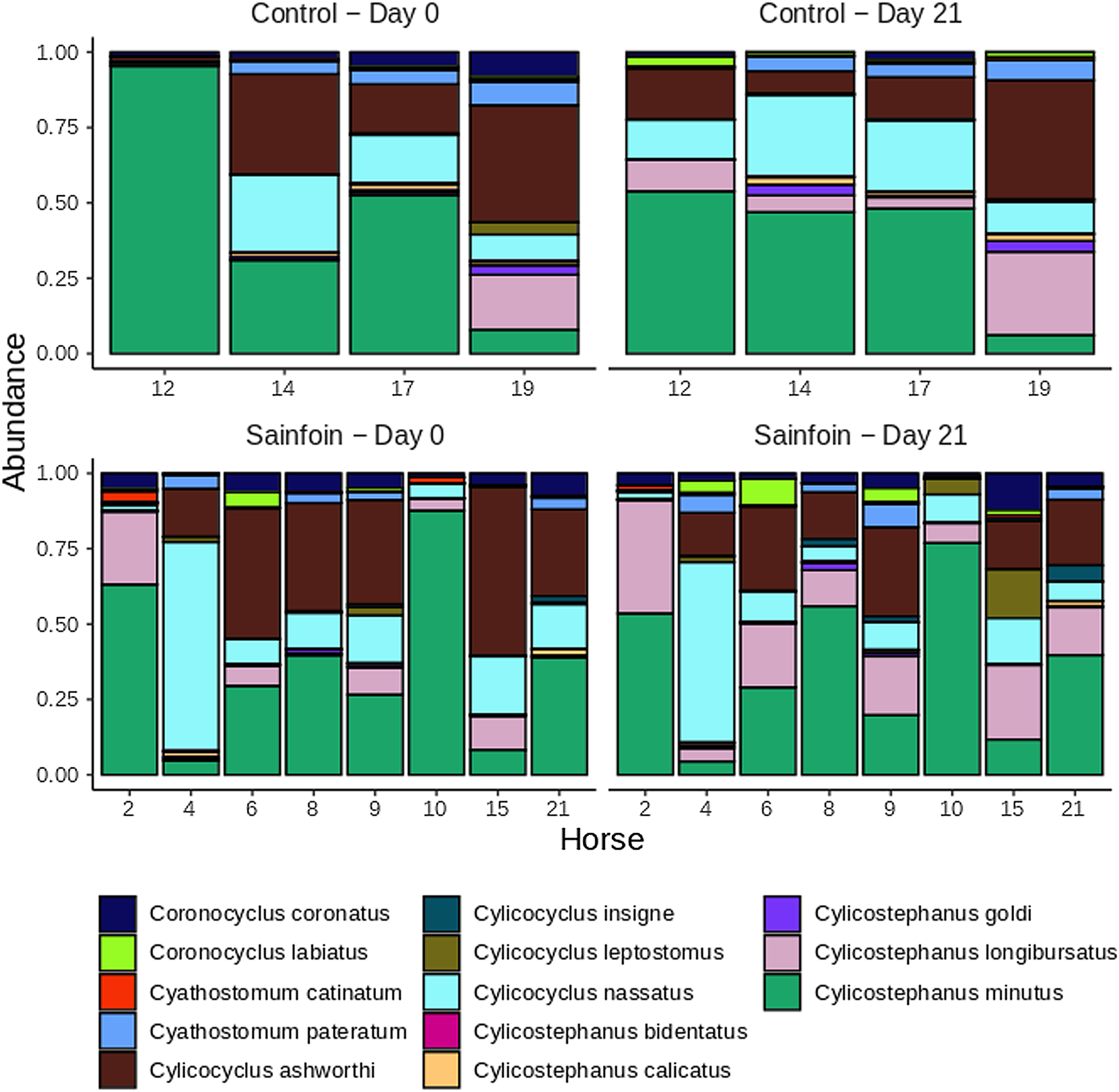
Fig. 3. Cyathostomin larval community structure estimated using the metabarcoding approach across days and groups. Relative abundance of cyathostomin species in control (upper panels) and sainfoin-fed horses (lower panels) on days 0 and 21 of the experiment. Data are from 12 horses with samples successfully amplified.
Analysis of species abundance trajectory from day 0 to day 21 was restricted to the top 4 most abundant species (Fig. 4). Cylicostephanus longibursatus contributed significantly fewer individuals than the other 3 species (differential 4th-root transformed count of 2.74 ± 1.27 relative to C. ashworthi, P = 0.03). No significant variation relative to the experimental diet was found for the top 4 most abundant species (P = 0.59–0.96, Fig. 4).
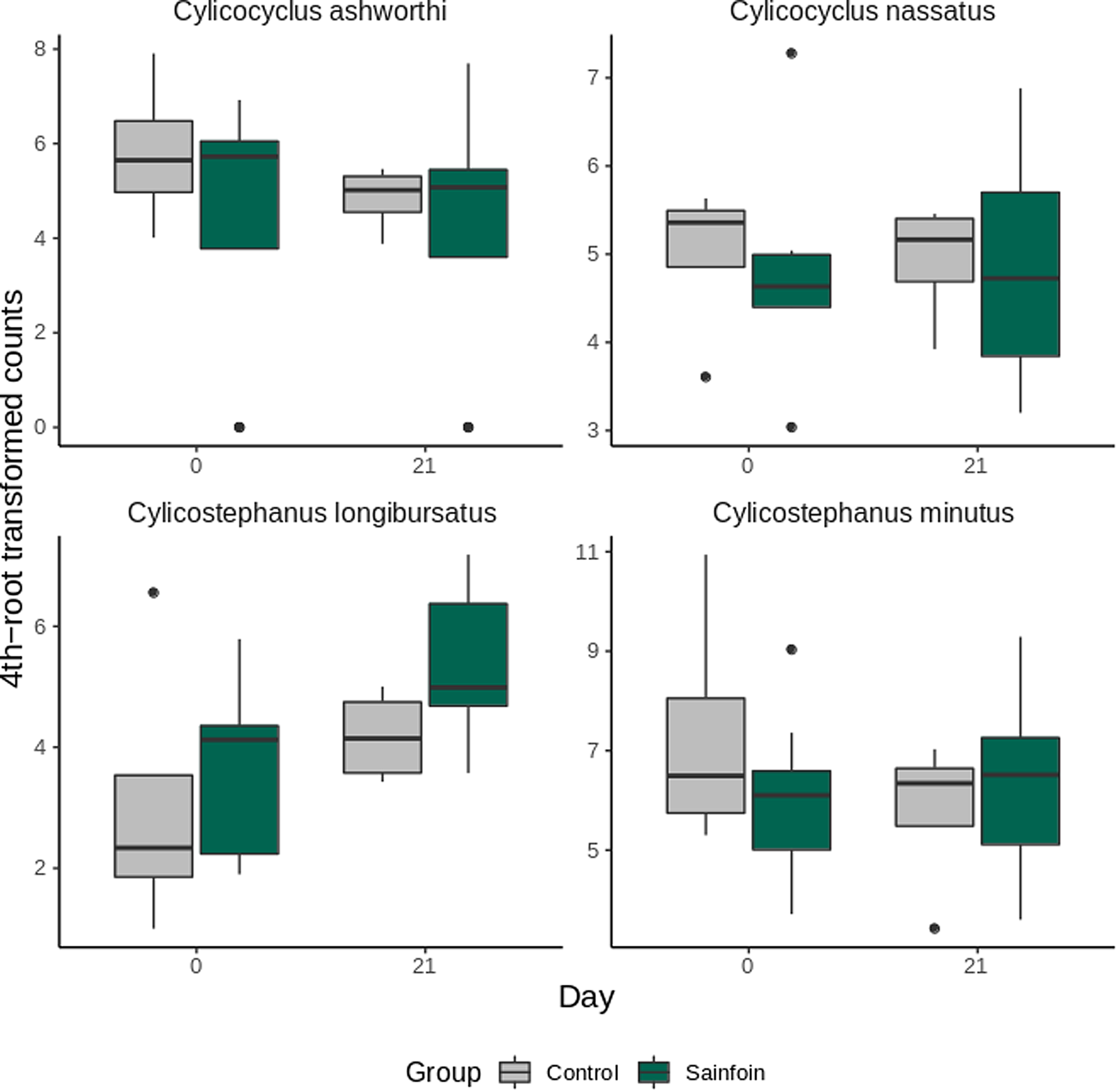
Fig. 4. Most abundant species estimated using the metabarcoding approach in horses from the control and sainfoin groups. Evolution of the 4 most abundant species in horses from the control and sainfoin groups between day 0 and day 21. Data are from 12 horses with samples successfully amplified.
Sainfoin effect on the ERP and ivermectin concentration in plasma following oral ivermectin treatment
Eggs reappeared 42 days after IVM treatment in the feces of both experimental groups (1 horse in each group). On day 78 (57 days after IVM treatment), sainfoin-fed horses excreted significantly more eggs than their counterparts fed with the control diet (P = 0.04, Fig. 5A). These excretion levels corresponded to FECR of 89.6% (75.4; 95.6) for the sainfoin group. This value was significantly lower than the FECR of the control group on the same day [95.8% (84.1; 98.9), Table 2]. However, the ERP was above 57 days in both groups. The plasmatic IVM concentration was also measured. The drug profiles followed a typical kinetic curve expected after an oral drench with a maximum IVM concentration (C max) recorded at 24 h in both groups. Importantly, the sainfoin diet negatively impacted the IVM concentration in plasma. These IVM concentrations were twice lower in horses of the sainfoin group when compared with those fed the control diet (Fig. 5B). This was observed at 24 and 48 h after IVM treatment (P = 0.001 and 0.006, respectively, Fig. 5B, Table 3). Consequently, the average AUC, reflecting the animal exposure to the IVM drug, was significantly lower in horses fed with the sainfoin diet (P < 0.001, Table 3) than in the control group.
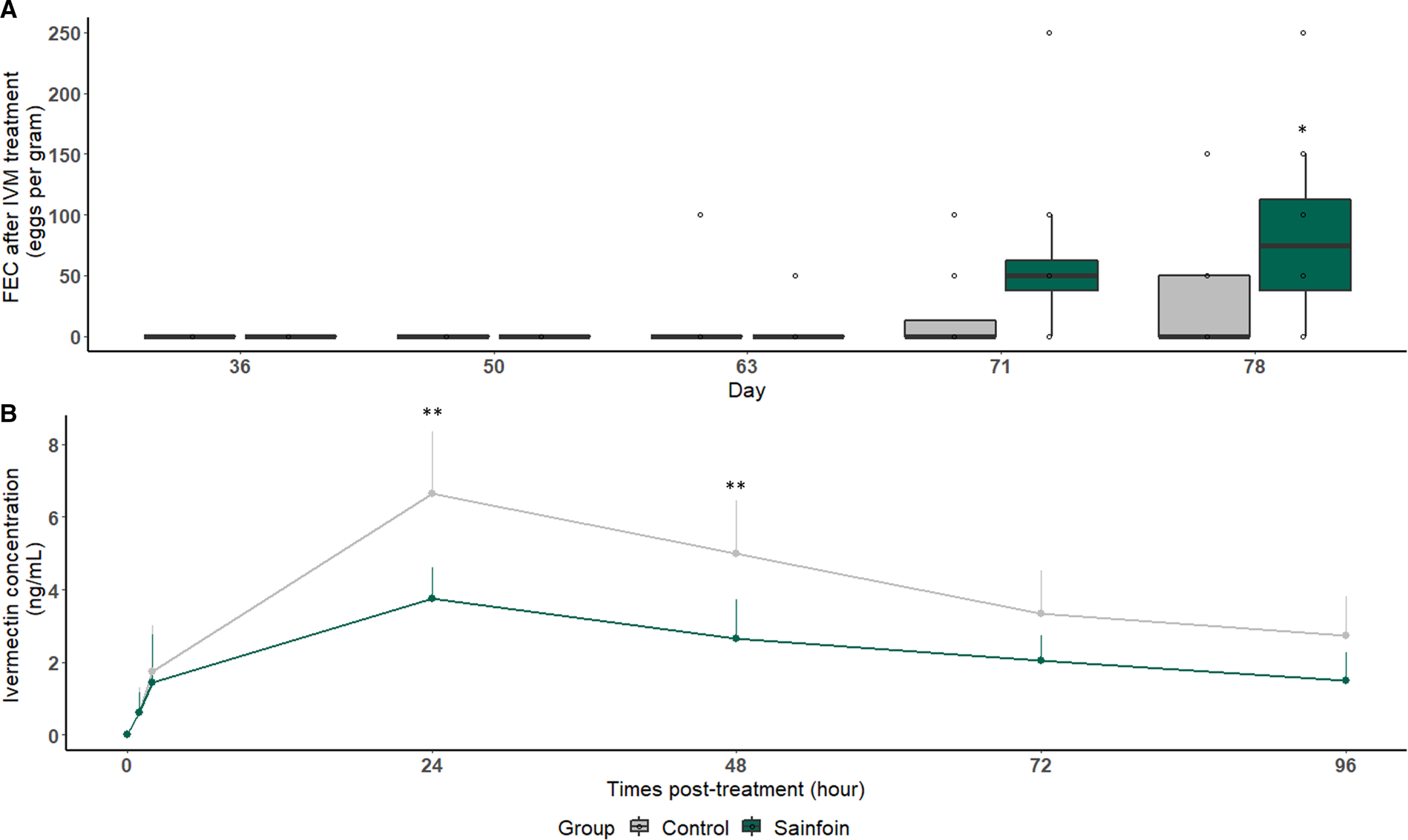
Fig. 5. Average fecal egg count (A) and plasma ivermectin concentration (B) measured after treatment. Average cyathostomin FEC (A) or IVM concentration in plasma (B) measured after IVM treatment is represented for horses receiving the control (light grey) or sainfoin (dark green) diet. *Statistically significant difference of FEC between the 2 groups at day 78 (P = 0.04) (A). **Statistically significant difference of average plasma IVM concentration between groups at 24 and 48 h post-treatment (P < 0.01) (B).
Table 2. Average FECR data (%; arithmetic mean with the 95% confidence interval) in horses receiving the control or sainfoin-enriched diet after IVM treatment

a After treatment.
b Not determined.
Table 3. Pharmacokinetic parameters of ivermectin in plasma of horses receiving the sainfoin-rich or control diet

The parameters were calculated from 7 time-point concentrations measured in each animal. Values are geometric mean ± s.e.M of 10 animals.
nsNot significant.
*** Statistically significant difference of kinetic parameters compared to control (P ⩽ 0.001).
a Maximum plasma concentration.
b Time of maximum concentration.
c AUC from 0 to the last time point.
Discussion
The present study evaluated the anthelmintic activity of a sainfoin diet as a possible alternative mode of control of cyathostomin populations in domestic horses and its interaction with IVM treatment. It provides evidence of a mild effect of dehydrated sainfoin pellets on larval development rate in vivo and their negative impact on the IVM pharmacokinetics. It also reports the first application of a nemabiome approach to study the effects of bioactive plants, which supports the lack of a systematic alteration of the cyathostomin larval community structure by sainfoin.
Our results agree with previous observations by Collas et al. (Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2018), who did not report any effect of dehydrated sainfoin pellets (3.6% DM condensed tannins in the diet) on strongyle FEC in horses. However, we observed a slight reduction of larval development rate in horses fed with sainfoin between day 14 and day 21 (9.7% vs 23.4% in the control group), i.e. the end of the distribution of experimental diets). On the contrary, Collas et al. (Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2018) demonstrated that sainfoin inhibited strongyle larvae development in vitro. This discrepancy between in vitro and in vivo studies may indicate that bioactive compounds, presumably condensed tannins (Hoste et al., Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin2006, Reference Hoste, Martinez-Ortiz-De-Montellano, Manolaraki, Brunet, Ojeda-Robertos, Fourquaux, Torres-Acosta and Sandoval-Castro2012), may not have reached sufficient levels in the gastrointestinal lumen of horses to affect the cyathostomin population. In small ruminants, it is suggested that a minimum threshold of 3–5% DM condensed tannins in the diet should be applied to observe an anthelmintic effect (Hoste et al., Reference Hoste, Martinez-Ortiz-De-Montellano, Manolaraki, Brunet, Ojeda-Robertos, Fourquaux, Torres-Acosta and Sandoval-Castro2012). Here, the concentration of condensed tannins in the diet was lower (i.e. 2.3% DM). Still, the proportion of dehydrated sainfoin pellets in the diet (i.e. 70% DM, as in Collas et al. (Reference Collas, Sallé, Dumont, Cabaret, Cortet, Martin-Rosset, Wimel and Fleurance2018)) could not have been increased without compromising the proper functioning of the horse's digestive system. We also relied on the same treatment duration as implemented in successful experiments in ruminants (Paolini et al., Reference Paolini, De La Farge, Prevot, Dorchies and Hoste2005; Heckendorn et al., Reference Heckendorn, Häring, Maurer, Zinsstag, Langhans and Hertzberg2006). A more extended period of the sainfoin diet may substantially affect larval development. However, the excess protein intake (i.e. 250% MADC requirements) for more than 3 weeks could have caused health problems in horses.
Our study also evaluated the potential interaction between the tannin-rich diet and an oral IVM treatment, and showed a substantial decrease of IVM concentration in plasma when the drug was given with tannin-rich diet. In a previous experiment conducted on lambs, lower IVM plasma concentration after an oral treatment was reported in animal fed with sainfoin compared to their counterparts fed with a control diet (Gaudin et al., Reference Gaudin, Simon, Quijada, Schelcher, Sutra, Lespine and Hoste2016). This lower IVM plasma concentration was associated with the chelation of the molecule by the tannins in vitro in a dose-dependent manner (Gaudin et al., Reference Gaudin, Simon, Quijada, Schelcher, Sutra, Lespine and Hoste2016). Similarly, the plasma IVM concentration was reduced in horses fed with sainfoin from 24 h onwards in our study. It is generally reported that the T max (time to peak plasma concentration) is 8 h for oral IVM treatment in horses (Gokbulut et al., Reference Gokbulut, Nolan and Mckellar2001; Saumell et al., Reference Saumell, Lifschitz, Baroni, Fusé, Bistoletti, Sagües, Bruno, Alvarez, Lanusse and Alvarez2017; Vyniarska et al., Reference Vyniarska, Ziółkowski, Madej-Śmiechowska and Jaroszewski2021). The measure at 8 h post-treatment was not practically feasible under our setting. In line with this lower IVM plasma concentration, measured fecal egg reduction was lower in the sainfoin group and egg excretion reappeared earlier in this group. Although the ERP was above 57 days in both groups, this observation is in favour of a decreased treatment efficacy associated with sainfoin.
This effect on IVM plasma concentration may be related to the presence of tannins in the intestinal fluid that could chelate the drug, and reduce the amount of drug available for intestinal absorption (Gaudin et al., Reference Gaudin, Simon, Quijada, Schelcher, Sutra, Lespine and Hoste2016). In addition, tannin-rich diets contain flavonoids, like the procyanidins (Quijada et al., Reference Quijada, Fryganas, Ropiak, Ramsay, Mueller-Harvey and Hoste2015), which are known to modulate the expression of ABC transporters (Morris and Zhang, Reference Morris and Zhang2006), like the P-glycoproteins (P-gps), pump the drug out of tissues and the organisms, lowering plasma IVM concentration (Bartley et al., Reference Bartley, McAllister, Bartley, Dupuy, Ménez, Alvinerie, Jackson and Lespine2009; Dupuy et al., Reference Dupuy, Alvinerie, Ménez and Lespine2010). Their inhibition increases IVM efficacy against susceptible H. contortus, a trichostrongylid of small ruminants (Kerboeuf et al., Reference Kerboeuf, Blackhall, Kaminsky and von Samson-Himmelstjerna2003; Bartley et al., Reference Bartley, McAllister, Bartley, Dupuy, Ménez, Alvinerie, Jackson and Lespine2009; Lespine et al., Reference Lespine, Ménez, Bourguinat and Prichard2012; Peachey et al., Reference Peachey, Pinchbeck, Matthews, Burden, Lespine, von Samson-Himmelstjerna, Krücken and Hodgkinson2017). There is evidence that polyphenols can decrease the parasite ATP-binding cassette (ABC) transporter activity or directly affect the parasite (Dupuy et al., Reference Dupuy, Larrieu, Sutra, Lespine and Alvinerie2003; Whitney et al., Reference Whitney, Wildeus and Zajac2013). In addition, differential expression of these genes was found between IVM-resistant and IVM-susceptible isolates of H. contortus (Mate et al., Reference Mate, Ballent, Cantón, Lanusse, Ceballos, Alvarez L and Liron2022) and cyathostomins (Peachey et al., Reference Peachey, Pinchbeck, Matthews, Burden, Lespine, von Samson-Himmelstjerna, Krücken and Hodgkinson2017) or Parascaris sp. (Janssen et al., Reference Janssen, Krücken, Demeler and von Samson-Himmelstjerna2015). But our results failed to show a positive interaction between the tannin-rich diet and oral IVM treatment and in our experimental conditions, it is impossible to determine the origin of the low plasma IVM concentration in horse fed tannin-rich diet. The set-up of an equine fermenter able to mimic the hindgut conditions similar to that available for the foregut (Strauch et al., Reference Strauch, Wichert, Greef, Hillegeist, Zeyner and Liesegang2017) could help validate this hypothesis in horses. In any case, these observations suggest that horse feed should be monitored in the field while measuring ERP, as variation in plant secondary metabolites like flavonoids may obscure the results or comparison across operations.
Because a slight difference in larval development rate was observed between the two groups, the metabarcoding approach was implemented to quantify the relative abundance of each species, with a specific interest for the Cyclicocyclus genus. Previous records using a reverse line blot assay found that members of that genus reappeared first following IVM or pyrantel treatment (van Doorn et al., Reference van Doorn, Ploeger, Eysker, Geurden, Wagenaar and Kooyman2014; Kooyman et al., Reference Kooyman, van Doorn, Geurden, Mughini-Gras, Ploeger and Wagenaar2016). Here, the sainfoin diet administered had no detectable effects on the cyathostomin larval community structure. This would be in line with the limited effects observed on other traits or with non-specific effects that apply to every species equally. To our knowledge, this is the first attempt to apply the metabarcoding approach to analyse the activity of a plant product in a parasite system. In addition, the use of mock communities of known composition highlighted two strands of improvement for this approach. Suboptimal taxonomic assignment was detected for the members of the Cyathostomum genus, with a high rate of uncertainty when attempting to distinguish between C. catinatum and C. pateratum in mock communities. This limitation adds up to the similarity between Cylicostephanus calicatus ITS-2 sequences and that of Coronocyclus coronatus worms, and to the presence of cryptic cyathostomin species in the community (Bredtmann et al., Reference Bredtmann, Krücken, Kuzmina, Louro, Madeira de Carvalho and von Samson-Himmelstjerna2019; Louro et al., Reference Louro, Kuzmina, Bredtmann, Diekmann, de Carvalho, von Samson-Himmelstjerna and Krücken2021). Additional markers like the mitochondrial cytochrome oxidase I (CO1) barcode may help increase the specificity of this approach. However, its higher rate of evolution provides a within-species resolution that may be more difficult to handle (Ramünke et al., Reference Ramünke, de Almeida Borges, von Son-de Fernex, von Samson-Himmelstjerna and Krücken2018).
This study confirmed the absence of effects of dehydrated sainfoin pellets in vivo on cyathostomin FEC and reported a weak impact on cyathostomin larval development. This is despite sainfoin pellets amounting to 70% of the horse diet. Applying a metabarcoding approach with mock communities revealed substantial margins for improvement in the taxonomic assignment. Still, it did not evidence any effect of sainfoin on the cyathostomin larval population structure. In addition, sainfoin significantly reduced IVM plasma concentration in horses leading to an accelerated reappearance of eggs in the feces. Altogether, sainfoin, administered as in this study, does not appear to be able to control cyathostomin in the field.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182022000853.
Data availability
The raw data files and associated R scripts are available under the following repository: https://github.com/Joshua-Malsa/Sainfoin-paper.git.
Acknowledgements
We are grateful to the GenoToul bioinformatics platform (https://doi.org/10.15454/1.5572369328961167E12) for providing computing and storage resources.
Author contributions
G. Fleurance, G. Sallé, B. Dumont and J. Malsa conceived and designed the study; G. Fleurance, L. Wimel, C. Dubois, E. Courtot, M. Boisseau, F. Guégnard, D. Serreau and J. Malsa gathered the data; A. Lespine and J-F. Sutra produced and analysed ivermectin plasma concentration data; J. Lluch, G. Annonay conducted the sequencing of cyathostomin ITS-2 amplicon; T. A. Kuzmina, M. Basiaga collected the samples used for the mock community preparation; G. Sallé performed the analyses of the cyathostomin ITS-2 amplicon sequencing data; J. Malsa performed statistical analyses; J. Malsa, G. Fleurance, G. Sallé, E. Courtot, T. A. Kuzmina, M. Basiaga, S. Dhorne-Pollet, N. Mach, A. Lespine drafted the paper; P. Gombault prepared the sainfoin pellets.
Financial support
This work benefited from the financial support of the Institut Français du Cheval et de l’Équitation (IFCE) and Institut Carnot France Future Élevage (F2E). Joshua Malsa is a grateful recipient of a joint Fond Eperon and IFCE fellowship.
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
The experimental procedure received approval from the French Ministry of Research under protocol number APAFIS#26140-2020062216271790v2.