Introduction
Diplozoidae Palombi, 1949 is a group of monogenean ectoparasites that mostly infect cyprinoid fishes (Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). This group is represented by two subfamilies – Diplozoinae Palombi, 1949 and Neodiplozoinae Khotenovsky, 1981. The typical feature of the members of Diplozoidae is the cross-shaped pattern of mature specimens, i.e. the adult body is formed by two permanently fused specimens (Khotenovsky, Reference Khotenovsky1985a, Reference Khotenovsky1985b; Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). While the adult of Diplozoinae species has four pairs of clamps as parts of two attachment organs, the adult of Neodiplozoinae is equipped with more than four pairs of clamps. The Neodiplozoinae include two genera – Afrodiplozoon Khotenovsky, 1981 and Neodiplozoon Tripathi, 1960, both reported only from Africa (Khotenovsky, Reference Khotenovsky1985b). The Diplozoinae include the following five genera: Diplozoon von Nordmann, 1832; Eudiplozoon Khotenovsky, 1984; Inustiatus Khotenovsky, 1978; Paradiplozoon Akhmerov, 1974 and Sindiplozoon Khotenovsky, 1981, all with a distribution previously considered to be limited to Palaearctic and Oriental zoogeographical ecoregions (Khotenovsky, Reference Khotenovsky1985b; Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). However, Paradiplozoon species have recently been described also from the African continent (e.g. Avenant-Oldewage et al., Reference Avenant-Oldewage, Le Roux, Mashego and Van Vuuren2014; Dos Santos et al., Reference Dos Santos, Van Vuuren and Avenant-Oldewage2015; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021). Adult diplozoids are located on fish gills and are obligatory blood-feeders, with some species, e.g. Eudiplozoon nipponicum, being important pathogens of cyprinoid fishes (Smyth and Halton, Reference Smyth and Halton1983; Khotenovsky, Reference Khotenovsky1985a; Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009; Rohlenová et al., Reference Rohlenová, Morand, Hyršl, Tolarová, Flajšhans and Šimková2011). Diplozoids are hermaphrodites with a monoxenous life cycle and usually lay one egg at a time, from which a free-living larva (oncomiracidium) hatches and actively seeks a host. After attaching to the host body, larvae move to the gills and grow into their post-oncomiracidial stage, termed diporpa. Two diporpae in their last stage of development (with three or four pairs of clamps already developed) fuse, and after reaching sexual maturity, copulate (Khotenovsky, Reference Khotenovsky1985a; Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009). The vitellarium, pharynx and suckers are located in the anterior parts of the fused specimens, and the haptor (comprising sclerotized hard clamps and central hooks), testes and ovaries are located in the posterior parts of the bodies of the fused specimens (Khotenovsky, Reference Khotenovsky1985a). While the clamps are the main attachment features in mature diplozoids, central hooks are used by the oncomiracidium for attaching to hosts (Bychowsky and Nagibina, Reference Bychowsky and Nagibina1959; Khotenovsky, Reference Khotenovsky1985a). The shapes and sizes of individual parts of the attachment apparatus are considered as the taxonomically most important characteristics, used for the species identification of diplozoids; however, some studies showed that the size of the clamps may correlate with the size of the host fish (e.g. Matějusová et al., Reference Matějusová, Koubková, Gelnar and Cunningham2002). Therefore, it has been suggested that the shape of individual haptoral sclerites represents the most important taxonomical feature for diplozoid identification (e.g. Matějusová et al., Reference Matějusová, Koubková, D'amelio and Cunningham2001b, Reference Matějusová, Koubková, Gelnar and Cunningham2002; Dos Santos and Avenant-Oldewage, Reference Dos Santos and Avenant-Oldewage2020).
There are also a few records of diplozoids on other fish species such as African Alestidae (Thomas, Reference Thomas1957; Paperna, Reference Paperna1969, Reference Paperna1979; Echi and Ezenwaji, Reference Echi and Ezenwaji2010), Cichlidae (Batra, Reference Batra1984; Yildirim et al., Reference Yildirim, Zeren, Genc, Erol and Konas2010), Gobiidae (Khotenovsky, Reference Khotenovsky1985b) and Cottidae (Nicoll, Reference Nicoll1924; Khotenovsky, Reference Khotenovsky1985b). Different levels of host specificity have been reported for diplozoid species. Some species are true generalists (i.e. species infecting a wide range of host species), e.g. Paradiplozoon homoion (Khotenovsky, Reference Khotenovsky1985b; Gelnar et al., Reference Gelnar, Koubková, Pláňková and Jurajda1994; Matějusová et al., Reference Matějusová, Koubková, Gelnar and Cunningham2002) and Paradiplozoon megan (Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021); however, most diplozoid species have been considered to be strictly host specific, e.g. Paradiplozoon moroccoensis (Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021), Paradiplozoon bingolensis (Civáňová et al., Reference Civáňová, Koyun and Koubková2013) and Paradiplozoon iraqensis (Al-Nasiri and Balbuena, Reference Al-Nasiri and Balbuena2016), as they are each currently reported only from a single-host species. In some studies, host specificity is applied as a taxonomically important criterion to describe new diplozoid species (e.g. Al-Nasiri and Balbuena, Reference Al-Nasiri and Balbuena2016), which has strongly biased new taxonomical reports. Although most published studies focused on diplozoids have been of a strictly taxonomic nature, i.e. aimed only at describing new species (e.g. Avenant-Oldewage et al., Reference Avenant-Oldewage, Le Roux, Mashego and Van Vuuren2014; Dos Santos et al., Reference Dos Santos, Van Vuuren and Avenant-Oldewage2015; Fan et al., Reference Fan, Meng, Bai, Xu and Wang2018; Arken et al., Reference Arken, Hao, Guo, Zhang, Rong, Kamal, Tian, Kadir and Yue2021; Cao et al., Reference Cao, Fu, Zou, Li, Wu, Wang, Blazhekovikj-Dimovska and Li2022) or re-describing previously known ones (e.g. Jirsová et al., Reference Jirsová, Ding, Civáňová, Jirounková, Ilgová, Koubková, Kašný and Gelnar2018, Reference Jirsová, Koubková, Jirounková, Vorel, Zhou, Ding, Gelnar and Kašný2021; Přikrylová et al., Reference Přikrylová, Mašová, Gelnar, Matla, Tavakol and Luus-Powell2018), the taxonomy of Diplozoidae is still problematic. Only a few recently published studies have tackled taxonomic issues relating to Diplozoidae by also employing a molecular phylogenetic approach in combination with morphological data; however, they unanimously suggest a need for major taxonomical revision within Diplozoidae (Civáňová et al., Reference Civáňová, Koyun and Koubková2013; Přikrylová et al., Reference Přikrylová, Mašová, Gelnar, Matla, Tavakol and Luus-Powell2018; Dos Santos and Avenant-Oldewage, Reference Dos Santos and Avenant-Oldewage2020; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021).
For the Middle East region, there are some generic checklists and faunistic reports that lack comprehensiveness and suggest that Iranian and Iraqi parasite faunas are only weakly investigated (e.g. Pazooki and Masoumian, Reference Pazooki and Masoumian2012; Abdul-Ameer and Mhaisen, Reference Abdul-Ameer and Mhaisen2013; Al-Nasiri, Reference Al-Nasiri2017; Mhaisen and Abdullah, Reference Mhaisen and Abdullah2017; Mhaisen, Reference Mhaisen2019); they reference outdated studies, do not investigate all regions and host taxa and often fail to consider the taxonomical reclassification of hosts. On the basis of the freshwater parasite fauna studies of fish in Turkey, 60 monogenean species were reported from native fish species (Öktener, Reference Öktener2003, Reference Öktener2014; Cinar, Reference Cinar2014). However, with respect to the diversity and distribution of Diplozoidae, the Middle Eastern region is only poorly investigated, with only a few studies focusing on Diplozoidae species diversity and the phylogenetic position of diplozoid species parasitizing native cyprinoids in Turkey (Civáňová et al., Reference Civáňová, Koyun and Koubková2013; Unal et al., Reference Unal, Innal, Civáňová, Stavrescu-Bedivan, Koubková, Ozmen and Gelnar2017).
To help fill this knowledge gap, the present study was designed (1) to investigate the distribution and diversity of diplozoids on cyprinoid host species in the Middle East, hypothesizing that endemic host species harbour endemic parasites; (2) to reveal the degree of host specificity in Diplozoidae species in the Middle East in light of high cyprinoid endemism in the region and (3) to investigate the position of Middle East species within diplozoid phylogeny, since the Middle East is considered as a historical crossroads for cyprinoid fishes (Coad, Reference Coad1996; Durand et al., Reference Durand, Tsigenopoulos, Ünlü and Berrebi2002).
Materials and methods
Sampling and species identification
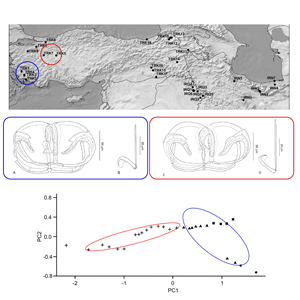
From 2018 to 2022, a total of 794 fish specimens were sampled at 32 localities in Iraq, Iran and Turkey using electrofishing (Supplementary Table 1, and Fig. 1). A total of 93 endemic cyprinoid species and one non-endemic species (Vimba vimba) were examined for the presence of diplozoid monogeneans (list of parasitized species is shown in Table 1). The species identification of the fish hosts was performed by means of morphology using relevant literature keys, and molecular data (i.e. complete cytochrome b sequences following Šanda et al. (Reference Šanda, Vukić, Choleva, Křížek, Šedivá, Shumka and Wilson2008)). The parasitological dissection protocol of Řehulková et al. (Reference Řehulková, Seifertová, Přikrylová, Francová, Scholz, Vanhove, Smit and Gelnar2018) was followed. The parasites were mounted on slides and preserved in a mixture of glycerine and ammonium picrate (GAP; Malmberg, Reference Malmberg1957). Prior to mounting, one of the parasite anterior parts was excised using fine needles and preserved in 96% ethanol for further DNA extraction. At least two whole specimens of selected Paradiplozoon species were flattened and preserved in 4% formaldehyde, and later stained by iron acetocarmine, dehydrated using a series of graded ethanol concentrations and mounted on slides in Canada balsam (following Georgiev, Reference Georgiev1986) to study the internal structures (i.e. soft body parts). Diplozoids fixed in GAP or Canada balsam were identified following available keys and taxonomic papers (Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009; Civáňová et al., Reference Civáňová, Koyun and Koubková2013; Avenant-Oldewage et al., Reference Avenant-Oldewage, Le Roux, Mashego and Van Vuuren2014; Dos Santos et al., Reference Dos Santos, Van Vuuren and Avenant-Oldewage2015; Al-Nasiri and Balbuena, Reference Al-Nasiri and Balbuena2016; Jirsová et al., Reference Jirsová, Ding, Civáňová, Jirounková, Ilgová, Koubková, Kašný and Gelnar2018, Reference Jirsová, Koubková, Jirounková, Vorel, Zhou, Ding, Gelnar and Kašný2021; Přikrylová et al., Reference Přikrylová, Mašová, Gelnar, Matla, Tavakol and Luus-Powell2018; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021) and using an Olympus BX51 light microscope (Olympus, Shinjuku, Japan) equipped with phase contrast.

Figure 1. Distribution map of diplozoid species collected during this study. Codes used in the map correspond to the locality codes in Table 1. IRQ: Iraq, IRN: Iran, TUR: Turkey
Table 1. List of collected Paradiplozoon species including host species, and epidemiological data

P, prevalence in %; I, minimum and maximum intensity of infection; A, mean abundance with confidence interval; N, host sample size; LocID, locality code; asterisk (*), new host records.
Parasite infection
All epidemiological parameters were calculated following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997). Those were: (1) prevalence, as the proportion of hosts infected by a given parasite species in the whole sample of examined hosts; (2) the intensity of infection, as the number of individual parasites on an infected host and (3) abundance, as the number of individual parasites on a given host regardless of the infection. Following the recommendation of Rózsa et al. (Reference Rózsa, Reiczigel and Majoros2000), confidence levels of 95% of mean abundance were calculated to properly infer epidemiological data. Using QPweb (http://www2.univet.hu/qpweb/qp10/index.php), bias-corrected and accelerated bootstrap confidence intervals were calculated due to the low number of hosts (Reiczigel et al., Reference Reiczigel, Marozzi, Fábián and Rózsa2019).
Morphometric data
Drawings of the attachment clamps and central hooks were made using an Olympus BX51 light microscope equipped with phase-contrast optics and a drawing tube. Drawings were redrawn and digitized using a graphic tablet (Wacom, Kazo, Japan) and Adobe Illustrator CS6 (Adobe, San Jose, USA). Measurements (in μm) were taken using digital image analysis (Stream Motion, version 1.9.2) (Olympus, Shinjuku, Japan) and are given as the mean followed by the range in parentheses (in μm). The terminology for haptoral structures follows that of Owen (Reference Owen1963), Khotenovsky (Reference Khotenovsky1985a) and Benovics et al. (Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021) with a little modification (see Fig. 2 for terminology). Type and voucher specimens were deposited in the Helminthological Collection of the Institute of Parasitology (IPCAS), Biology Centre of the Czech Academy of Sciences, České Budějovice, Czech Republic. To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN 2012), details of the new species have been submitted to ZooBank.

Figure 2. Scheme of general structures of diplozoid clamps: (a) anterior half of median plate; (b) trapeze spur; (c) anterior joining sclerite; (d) anterior clamp jaw; (e) lateral sclerite; (f) posterior clamp jaw; (g) distal posterior joining sclerite; (h) proximal posterior joining sclerite; (i) tendon guiding termination and (j) posterior half of median plate.
In addition, to assess intraspecific morphological variability in the morphometrical characteristics of the haptoral elements of collected Paradiplozoon species, the following analyses were performed. To remove the effect of host body size on parasite body size, linear regression was used with the host's standard length as the independent variable and haptoral element measurements as a dependent variable; the residuals from linear regression were used for further analysis. Principal component analysis (PCA) was used to differentiate phenotype variability among selected generalist Paradiplozoon species. All statistical analyses were performed and visualized by R v. 4.1.3 using the packages ‘FactoMineR’, ‘ggplot2’, ‘ggbiplot’ and ‘ggpubr’.
DNA extraction, amplification and sequencing
Prior to DNA extraction, the parasite tissues preserved in 96% ethanol were dried using a vacuum centrifuge. DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) was used for extraction following the manufacturer's protocol. Primers D (5′-GGCTYRYGGNGTCGATGAAGAACGCAG-3′) and B1 (5′-GCCGGATCCGAATCCTGGTTAGTTTCTTTTCCT-′3) (Baehellerie and Qu, Reference Baehellerie, Qu and Hyde1993) were used to amplify the complete internal transcribed spacer 2 (ITS2) region, and the polymerase chain reaction (PCR) amplification protocol following Matějusová et al. (Reference Matějusová, Koubková, D'amelio and Cunningham2001b). For fish tissue, the amplification of the entire cytochrome b gene was performed using forward primer GluF (5′-AACCACCGTTGTATTCAACTACAA-3′) and reverse primer ThrR (5′-ACCTCCGATCTTCGGATTACAAGACCG-3′) (Machordom and Doadrio, Reference Machordom and Doadrio2001), following the amplification protocol according to Šanda et al. (Reference Šanda, Vukić, Choleva, Křížek, Šedivá, Shumka and Wilson2008). PCR products were checked on 1% agarose gel and then purified using the ExoSAP-IT enzyme standard protocol (Amplia, Bratislava, Slovakia). Sequencing was carried out at the Macrogen Service Centre (Amsterdam, Netherlands) using the amplification primers. Newly acquired sequences of Paradiplozoon specimens and fish hosts were deposited in the GenBank database (see Table 2 for accession numbers).
Table 2. List of Diplozoidae species included in phylogenetic analyses

Host species, locality of collection and accession number from GenBank for ITS2 sequence of each parasite's species are included. NA, data are not available, newly acquired sequences are marked by asterisks (*).
Phylogenetic analyses
In addition to newly obtained ITS2 sequences, 49 orthologue sequences of 26 diplozoid species were retrieved from GenBank (Table 2) to infer phylogenetic relationships between diplozoid species from the Middle Eastern region and diplozoid species from other regions. The sequence alignment was built using fast-Fourier transform in MAFFT and applying the G-INS-i iterative refinement algorithm (Katoh et al., Reference Katoh, Misawa, Kuma and Miyata2002). All the aligned sequences were trimmed to unify their length. To find the best substitution model, ModelGenerator v. 0.85 (Keane et al., Reference Keane, Creevey, Pentony, Naughton and Mclnerney2006) was used, and the best model based on the Bayesian information criterion was selected for further phylogenetic analysis. Intraspecific genetic distances were computed using sequences from the ITS2 region. Uncorrected pairwise distances were calculated in MEGA 11 (Tamura et al., Reference Tamura, Stecher and Kumar2021). Maximum-likelihood (ML) and Bayesian inference (BI) phylogenetic reconstructions were computed using iQtree v. 2.1 (Nguyen et al., Reference Nguyen, Schmidt, Von Haeseler and Minh2015) and MrBayes v. 3.2 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012), respectively. The best ML tree was selected from 2000 iterations with support for the branching pattern validating through 2000 bootstrap pseudo-replicates. The BI tree was constructed using the Metropolis-coupled Markov chain Monte Carlo algorithm with two parallel runs comprising four concurrent chains (one cold and three hot) running for 2 000 000 generations, with trees sampled every 100 generations. The initial 30% of trees were discarded as ‘burn-in’, after checking the standard deviation split frequency fell below 0.01. The convergence of evolutionary model parameters was then checked by Tracer v. 1.7.1 (Rambaut et al., Reference Rambaut, Drummond, Xie, Baele and Suchard2018). The final phylograms were rooted using Octomacrum europaeum of Octomacridae, as a representative species of a family phylogenetically proximal to Diplozoidae (Sicard et al., Reference Sicard, Desmarais and Lambert2002).
Results
Diversity and infection load of Paradiplozoon in the Middle East
A total of four Paradiplozoon species were collected from 48 out of 93 investigated host species in three Middle Eastern countries (Table 1). These included P. bingolensis, Paradiplozoon bliccae and P. homoion, as well as Paradiplozoon koubkovae n. sp., identified as a new species for science. Paradiplozoon bingolensis was collected only from Cyprinion macrostomum at two localities in Iraq and Cyprinion kais at one locality in Turkey. Paradiplozoon bliccae was collected only from cyprinoid species in Turkey and herein, it was recorded on six new host species (Table 1). The 100% prevalence of P. bliccae was found on Luciobarbus kottelati from the Çine River (Turkey). However, the highest maximum intensity of infection, and mean abundance by P. bliccae were recorded on Petroleuciscus ninae (i.e. I = 1–20, A = 2.9) and Barbus xanthos (I = 1–19, A = 5), respectively, at the same locality. Paradiplozoon homoion exhibited the widest host range among the collected Paradiplozoon species and was found on 36 fish (33 new host records, see Table 1) species belonging to eleven cyprinoid genera. The 100% prevalence of P. homoion was recorded on Alburnoides aff. damghani and Capoeta buhsei from Iran; Alburnus sellal from Iraq and Alburnus carianorum, Alburnoides kosswigi, Barbus tauricus, Capoeta damascina and Squalius cii from Turkey. Paradiplozoon homoion reached the highest mean abundance on Alburnus chalcoides (from Iran) followed by B. tauricus, S. cii, A. carianorum (from Turkey) and Chondrostoma regium from Iraq (Table 1). The last species, P. koubkovae n. sp., was collected from two localities in Iran and Turkey (Table 1), where only one specimen was found on Luciobarbus capito (Iran) and four specimens were collected from Capoeta capoeta (Turkey).
Morphology
Paradiplozoon bliccae (Reichenbach-Klinke, 1961)
Description: With soft body characters of the species. Attachment apparatus (haptor) consisting of four pairs of clamps and a pair of central hooks lying on ventral side of each haptor. Clamps arranged bilaterally in two parallel longitudinal rows, with their openings directed ventrally. First pair of clamps slightly smaller than other pairs. Each clamp composed of sclerites in configuration typical for species of Paradiplozoon (see Fig. 2). Median plate J-shaped, with median groove perforated by lacunae throughout most of its length. Anterior half of median plate distally elongated into rectangular trapeze spur (not trapezoid or fishtail-shaped) and directly connected with anterior jaw. Arches (left, right) of anterior jaw proximally fused together, forming converse T-shaped junction with median plate. Posterior half of median plate with tendon guiding termination comprising a rounded collar-shaped structure distally supported by lightly sclerotized triangular enlargement. Posterior half of median plate connected to arches of posterior jaw by two posterior joining sclerites; proximal posterior joining sclerite twice as long as distal posterior joining sclerite, with median groove. Anterior jaw comprising two (left, right) relatively thin arches; each with median groove perforated by lacunae throughout 0.7 of its length, proximal larger half arched (by its convexity ventrolaterally), distal minor half recurved into wing-shaped part. Wing-shaped part with inwardly directed spur and small joint socket for articulating head of lateral sclerite. Posterior jaw composed of two (left, right) smooth arches; each with thickened convex side and slightly bifurcated end to give bone-like appearance. Two lateral sclerites bilaterally connecting anterior and posterior jaws; each about 0.5–0.75 of length of posterior jaw arch. Central hooks situated in proximity of inner margin of most posterior pair of clamps (i.e. pair of clamp I).
Remarks
Paradiplozoon bliccae is the only known species of the genus possessing proximally fused arches of anterior clamp jaw, forming converse T-shaped junction with median plate. Two morphological variants of P. bliccae were recognized: P. bliccae variant A1 (Fig. 3A and B) collected from B. xanthos, Capoeta aydinensis, Ladigesocypris ghigii, L. kottelati, P. ninae, Squalius fellowesii and Vimba mirabilis, and P. bliccae variant A2 (Fig. 3C and D) collected from V. vimba. The uncorrected P-distance between P. bliccae variants in ITS2 sequences was 0.7%, and the distances within variants A1 and A2 were 2 and 0.5%, respectively (Supplementary Table 2).
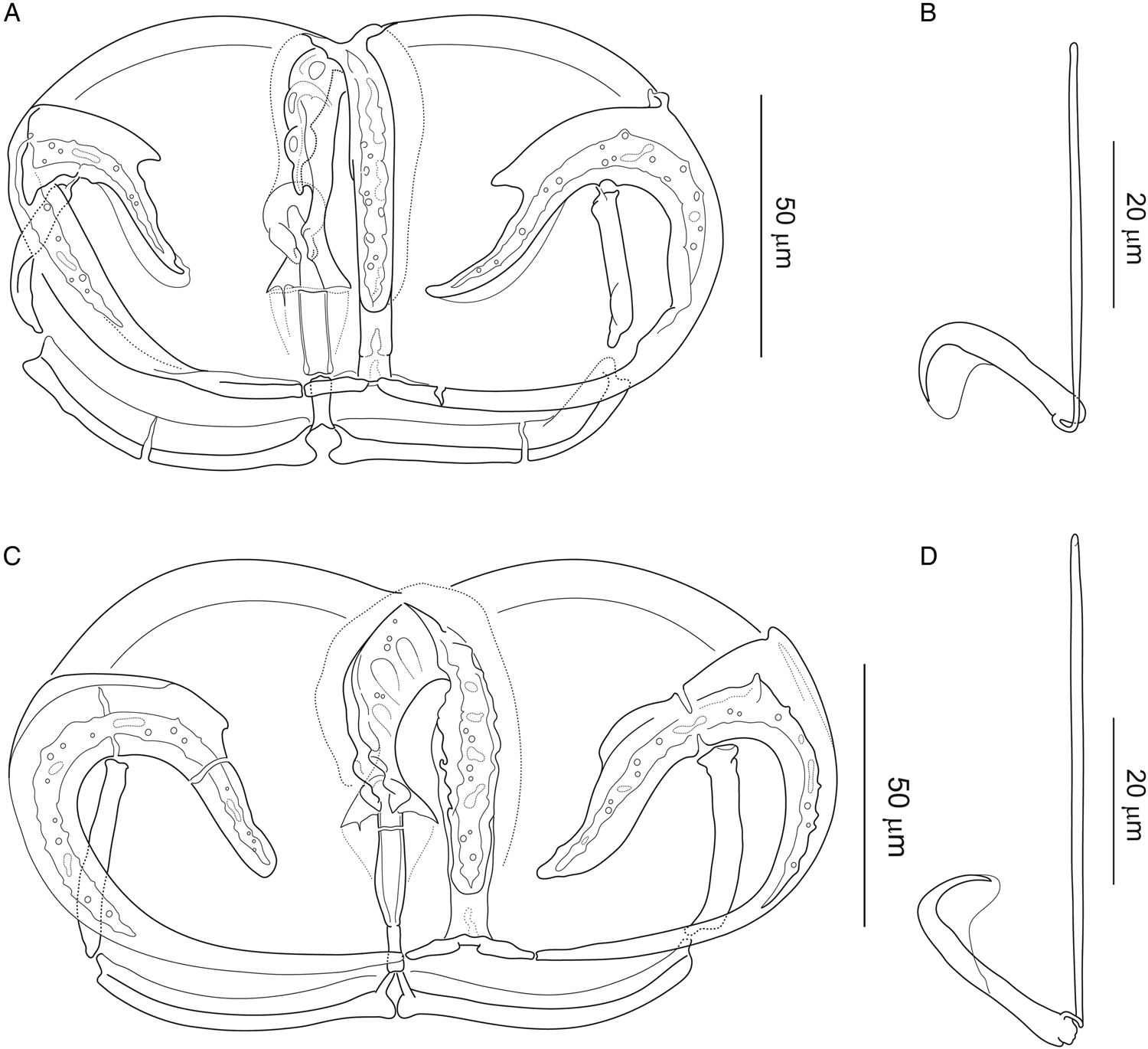
Figure 3. Attachment clamp III and central hook of Paradiplozoon bliccae (Reichenbach-Klinke, 1961) from (A, B) Luciobarbus kottelati (Turkey) (IPCAS, M-300/4) and (C, D) from Vimba vimba (Turkey) (IPCAS, M-300/9).
Figure 3 provides the morphological differentiation of the two forms: (1) central hooks are generally larger, with significantly longer handle in P. bliccae variant A2; (2) T-shaped junction of median plate and anterior jaw (i.e. trapeze spur + proximal ends of arches of anterior jaw) is more massive in P. bliccae variant A2 and (3) proximal posterior joining sclerite in P. bliccae variant A1 appears to be less sclerotized than that in variant A2. Respective data for variants A1 and A2 (i.e. host and locality records, localities records, measurements and drawings of sclerites) are presented below.
The majority of P. bliccae specimens collected (i.e. specimens collected from B. xanthos, L. ghigii and P. ninae) were diporpae or were in a recently fused ‘just married’ state without fully developed sclerites in the haptors, and, therefore, were not suitable for investigating intraspecific morphological variability. The remaining specimens were adult specimens of P. bliccae of the variant A1. For the study of morphometrical variation among host species, we used the specimens of P. bliccae variant A1 parasitizing 3 host species (C. aydinensis, L. kottelati and S. fellowesii) and specimens of P. bliccae variant A2 parasitizing V. vimba. Using PCA, the first axis (PC1) and the second axis (PC2) contributed 89.50 and 7.52% to explaining the variation in the dataset, respectively. Table 3 shows the correlations between morphometric characteristics and PC1 and PC2. By plotting measurements of the haptoral elements (i.e. clamps and hooks) of P. bliccae specimens of variants A1 and A2 in factorial space (PC1 and PC2) (Fig. 4), the two variants were separated according to their geographical distribution (variant A1: south-eastern Aegean drainage area, variant A2: south-western Black Sea).

Figure 4. Two variants of Paradiplozoon bliccae (A1: blue ellipse and A2: red ellipse) in their morphometrical space based on PCA: (●) Capoeta aydinensis, (▴) Luciobarbus kottelati, (■) Squalius fellowesii and (+) Vimba vimba.
Table 3. First 2 factorial axes produced by PCA were compared to the morphometric parameters using Pearson's correlation coefficients

Those parameters for which the correlation coefficients, between original parameters and factorial axes, were statistically significant are given in bold.
Paradiplozoon bliccae variant A1 (Fig. 3A and B)
Hosts, localities and specimens studied (hologenophores): Barbus xanthos (locality TRK1) (IPCAS, M-300/2), C. aydinensis (locality TRK1) (IPCAS, M-300/3), L. ghigii (locality TRK2) (IPCAS, M-300/7), L. kottelati (locality TRK1) (IPCAS, M-300/4), P. ninae (locality TRK1 and TRK2) (IPCAS, M-300/5), S. fellowesii (locality TRK3) (IPCAS, M-300/6) and V. mirabilis (locality TRK3) (IPCAS, M-300/8).
Measurements [based on ten adult specimens (20 haptors); in μm]: Size of clamps (width × height): clamp I = 117 (102–128) × 87 (78–93); clamp II = 140 (127–152) × 88 (82–95); clamp III = 139 (106–159) × 84 (71–99) and clamp IV = 128 (100–161) × 80 (67–98). Central hooks: sickle length 22 (21–23); handle length 49 (47–51).
Paradiplozoon bliccae variant A2 (Fig. 3C and D)
Host, locality and specimen studied (hologenophores): Vimba vimba (locality TRK5) (IPCAS, M-300/9).
Description: With characters of species [based on measurements of four adult specimens; i.e. eight haptors]: Size of clamps (width × height): clamp I = 117 (109–127) × 81 (70–89), clamp II = 147 (131–164) × 84 (75–101); clamp III = 156 (133–176) × 84 (75–98); clamp IV = 158 (139–170) × 85 (73–111) μm. Central hooks: sickle length 24 (23–26); handle length 58 (54–62).
Paradiplozoon koubkovae Řehulková, Nejat and Benovics n. sp.
Type host: Luciobarbus capito (Güldenstädt, 1773).
Type locality: Ghezel Ozan River, Iran (36°47′16″E, 49°07′14″E).
Other host and locality: Capoeta capoeta Levin, Prokofiev & Roubenyan, 2019, B-20 Canal at Aralık, a drainage of Arax River, Turkey (39°54′26″N, 44°30′28″E).
Site on host: Gill lamellae.
Specimens studied: Holotype, paratype, two hologenophores (IPCAS M-773/1); two vouchers and one hologenophore from C. capoeta (IPCAS M-773/2).
ZooBank registration: The Life Science Identifier (LSID) for the new name Paradiplozoon koubkovae n. sp. Řehulková, Nejat et Benovics is urn:lsid:zoobank.org:pub:29EED971-8DDB-450E-BF7C-132E534A142F.
Etymology: This species is named after Dr Božena Koubková to honour her contributions to knowledge of diplozoid fauna.
Description: Body X-shaped, comprising two adult individuals fused in permanent copula. Fusion delimiting forebody and hindbody in each specimen; forebody 2750 (n = 1) long, 826 (n = 1) wide; hindbody 1710 (n = 1) long. Tegument with small to inconspicuous annular transverse folds or ridges, less prominent to absent in hindbody.
Forebody pyriform, densely filled with vitelline follicles. Mouth relatively large, crescent-shaped, subterminal on ventral side, opening into buccal cavity. Buccal cavity comprising two muscular buccal suckers located bilaterally close to dorsal surface; each 110 (n = 1) in diameter. Pharynx immediately following buccal cavity; 91 (n = 1) long and 89 (n = 1) wide. Intestinal caecum branched, running posteriorly from pharynx into hindbody, ending blindly close to anterior margin of haptor. Transversal diverticula poorly defined due to vitellarium.
Hindbody without rounded or lobed widening just anterior to the haptor, comprising anteriorly situated reproductive organs lacking sclerotized parts and distally located attachment organ (haptor). Haptor without medial dilatation, composed of four pairs of clamps and one pair of central hooks lying on ventral side of each haptor. Clamps arranged bilaterally in two parallel longitudinal rows. First and fourth pairs of clamps a little smaller than other clamps (Fig. 6A). Size of clamps (based on measurements of six adult specimens/eight haptors; width × length): clamp I = 109 (127–98) × 85 (78–95); clamp II = 114 (138–97) × 82 (98–73); clamp III = 110 (127–97) × 78 (90–70); clamp IV = 96 (116–70) × 71 (85–53). Clamps relatively massive; each with well-developed sclerites in configuration typical for species of Paradiplozoon (see Fig. 2). Median plate U-shaped, with median groove on its outer side, perforated by lacunae throughout most of its length. Anterior half of median plate with clearly visible lacunae along the inner edges of the groove, distally enlarged into fishtail-shaped trapeze spur and connected with the anterior clamp jaw by one anterior joining sclerite. Anterior joining sclerite large, cube-shaped (often widened in the proximal part to the shape of an anvil). Posterior half of median plate appearing to be wider than the anterior half, distally slightly narrowing before expanding into tendon guiding termination. Tendon guiding termination comprising collar-shaped part arising subterminally from its outer side and trapezial terminal extension protruding distally from beneath collar (i.e. on inner side of median plate). Posterior half of median plate connected with posterior clamp jaw by two posterior joining sclerites, both tapering distally: one proximal joining sclerite wider than longer, with a knob in middle of its distal side; one distal joining sclerite longer than wider, with conspicuous median groove on its outer surface. Anterior jaw comprising two (left, right) massive arches; each with median groove perforated by lacunae throughout most of its length, proximal larger half arched (by its convexity ventrolaterally), distal minor half recurved into wing-shaped part. Wing-shaped part with well-developed inwardly directed spur and small joint socket for articulating head of the lateral sclerite. Posterior jaw composed of two (left, right) massive smooth arches; each with thickened convex side proximally enlarged into a rounded process. Two lateral sclerites bilaterally connecting anterior and posterior jaw arches; each about 0.5 of length of the posterior jaw arch, with a conspicuous ridge. Central hooks situated in proximity of inner margin of the most posterior pair of clamps (i.e. clamp I); sickle length 27 (29–25; n = 8); handle length 57 (50–64; n − 8).
Remarks
Although only six haptors were available for morphological study, the diplozoid specimen collected from L. capito (Iran) and C. capoeta (Turkey) was easily assigned to Paradiplozoon on the basis of the absence of prominent dilatation of prehaptoral region in the posterior end of the body. Comparison of the morphological details of clamp sclerites presently known for P. koubkovae n. sp. with those of previously described species of Paradiplozoon suggests that the specimen reported here represents an undescribed species. The basic configuration of the clamp sclerites indicates that P. koubkovae n. sp. (Figs 5 and 6) is morphologically close to Paradiplozoon vaalense reported on Labeo umbratus in South Africa by Dos Santos et al. (Reference Dos Santos, Van Vuuren and Avenant-Oldewage2015). On the basis of the morphology of clamp sclerites suggested in the drawings by these authors, the following characters are common to both species: anterior half of the median plate is distally enlarged into a trapeze spur; anterior joining sclerite is relatively massive and cube-shaped; posterior half of median plate is connected with posterior jaw by two posterior joining sclerites; arches of posterior jaw are relatively massive. Paradiplozoon koubkovae n. sp. differs from P. vaalense by having (1) large, fishtail-shaped trapeze spur (vs short, rectangular trapeze spur in P. vaalense); (2) proximal posterior joining sclerite longitudinally shorter than distal one (proximal and distal posterior joining sclerites are of similar length in P. vaalense); (3) proximal posterior joining sclerite with thickened lateral margins and a medial knob arising from its distal side (compared with pair of match-shaped thickenings passing parallel through middle in P. vaalense) and (4) slightly larger central hooks (sickle length 27 and handle length 55 vs 19 and 43, respectively, in P. vaalense).

Figure 5. Attachment clamp III (A) and central hook (B) of Paradiplozoon koubkovae n. sp. (IPCAS M-773/1) from Luciobarbus capito (Iran).

Figure 6. Paradiplozoon koubkovae Řehulková, Nejat et Benovics n. sp. from Luciobarbus capito. Phase-contrast micrographs of (A) haptor and (B) third clamp.
Dos Santos et al. (Reference Dos Santos, Van Vuuren and Avenant-Oldewage2015) stated that in P. vaalense, two small additional sclerites are sometimes observed between the anterior joining sclerite and anterior jaw arches. In one attachment clamp of P. koubkovae n. sp., the pointed proximal ends of the anterior jaw arches look like two additional sclerites. The two small additional sclerites reported by Dos Santos et al. (Reference Dos Santos, Van Vuuren and Avenant-Oldewage2015) in P. vaalense may actually represent proximal ends of the anterior jaw arches, but further investigation is needed to verify this suggestion.
Comparison of P. koubkovae n. sp. with another phylogenetically close species, Paradiplozoon ichthyoxanthon in Avenant-Oldewage et al. (Reference Avenant-Oldewage, Le Roux, Mashego and Van Vuuren2014) (see also Fig. 7), was not possible because the original drawing of the clamp sclerites of the latter species lacks the detailed information required for proper species differentiation. However, scanning electron micrographs of the clamp sclerites of P. ichthyoxanthon reported by Dos Santos et al. (Reference Dos Santos, Dzika and Avenant-Oldewage2019) clearly show that the anterior end of the median plate is not extended into the trapeze spur, which is at least one of the features that distinguishes this species from P. koubkovae n. sp. However, the scanning electron micrographs of clamp sclerites of P. ichthyoxanthon reported by Dos Santos et al. (Reference Dos Santos, Dzika and Avenant-Oldewage2019) show that the morphological configuration of sclerites connecting the posterior clamp jaws with the median plate is similar to that of P. koubkovae n. sp., i.e. comprising proximal posterior joining sclerite with tri-forked distal margin and distal posterior joining sclerite slightly narrowing distally. In addition, unlike P. vaalense, both former mentioned species possess relatively large trapeze spur resembling a fish tail. However, P. koubkovae n. sp. can be differentiated from P. ichthyoxanthon by having the collar-shaped part of the tendon guiding termination with long wings (vs short wings in P. ichthyoxanthon; cf. Fig. 6I in Dos Santos et al., Reference Dos Santos, Dzika and Avenant-Oldewage2019).

Figure 7. Phylogenetic tree of 95 ITS2 sequences of 27 diplozoid species reconstructed by BI. The numbers at each node represent posterior probabilities and bootstrap support values, resulting from BI and ML analyses, respectively. Dashes indicate posterior probability below 0.70 and bootstrap value below 50. Numbers in the brackets indicate the number of sequences in each collapsed branch. Coloured areas and letters are referred in results.
Phylogenetic relationships within the Diplozoidae
The final alignment included 96 ITS2 sequences (including outgroup) and spanned 634 unambiguous nucleotide positions. TVM + G was selected as the best DNA substitution model and used in BI and ML analyses. BI and ML trees showed the same topologies. The resulting BI phylogenetic tree including posterior probabilities and bootstrap support values is presented in Fig. 7. To observe genetic intraspecific variability, the final dataset included all representative sequences for Paradiplozoon specimens from the investigated parasite populations in the Middle East (P. bliccae, P. homoion and P. bingolensis). No intraspecific genetic variability was found among P. bingolensis. Also, no intraspecific genetic variability was observed among P. homoion specimens from various host species, despite this species' remarkable recorded host range. However, substantial intraspecific genetic variability, as well as morphological intraspecific variability, were observed among P. bliccae individuals (see remarks on P. bliccae, Supplementary Table 2).
Phylogenetic reconstruction revealed six major clades of the Diplozoidae species (Fig. 7). According to the phylogenetic tree, Paradiplozoon was polyphyletic, and four Paradiplozoon species collected from Middle East cyprinoid species (Fig. 7, species shown in red colour) were placed in two divergent clades. Clade A formed a well-supported monophyletic group and included 13 Paradiplozoon species and Diplozoon paradoxum. The positions of species in the subclade including P. megan, Paradiplozoon helleni, P. koubkovae n. sp., P. ichthyoxanthon and P. vaalense were not fully resolved within clade A; however, these five species were divided into two well-supported groups. Paradiplozoon megan and P. helleni formed a monophyletic group and P. koubkovae n. sp. from the Middle East had the sister position to African P. vaalense and P. ichthyoxanthon. Clades B, C and D each represented different diplozoid genera. Eudiplozoon spp. (clade C) and Inustiatus spp. (clade D) formed a monophyletic group, which was in sister position to the monophyletic group including Sindiplozoon spp. (clade B) and Paradiplozoon spp. with D. paradoxum (clade A). Clade E included three African species and P. bingolensis from the Middle East. Interestingly, Afrodiplozoon polycotyleus was in sister position to P. bingolensis (the Middle East) and Paradiplozoon krugerense (Africa); however, its phylogenetic position was unresolved by bootstrap support values. All the species from clade F were from East Asia. The phylogenetic relationships between clade E, clade F and the big lineage including clades A, B, C and D were unresolved from the current dataset.
Discussion
A total of 16 diplozoid species were previously reported from 30 Middle Eastern cyprinoid species (Pazooki and Masoumian, Reference Pazooki and Masoumian2012; Mhaisen and Abdul-Ameer, Reference Mhaisen and Abdul-Ameer2014; Öktener, Reference Öktener2014; Al-Nasiri and Balbuena, Reference Al-Nasiri and Balbuena2016). Herein, we reported four diplozoid species from 48 cyprinoid species and 32 localities in Iran, Iraq and Turkey increasing the total number of diplozoid species in Middle East to 17. Among Middle Eastern diplozoids, P. bingolensis is one of the relatively recently described species, originally recorded on Garra rufa in Turkey (Civáňová et al., Reference Civáňová, Koyun and Koubková2013). In the present study, this species was also recorded on C. macrostomum in Iraq, and C. kais in Turkey, expanding its potential distribution range, and adding new host records for this parasite species. Since both Garra and Cyprinion are phylogenetically related taxa belonging to the highly diversified Cyprinidae (Tan and Armbruster, 2018), we can assume that P. bingolensis might represent a generalist parasite species restricted only to phylogenetically related cyprinids, in contrast to the original proposal of its strict host specificity (Civáňová et al., Reference Civáňová, Koyun and Koubková2013). The next diplozoid species reported on Middle Eastern cyprinoids was P. bliccae, previously found only in Iraq on C. macrostomum and Cyprinus carpio (Al-Nasiri, Reference Al-Nasiri2009); however, the presence of P. bliccae on C. carpio is not usual. Surprisingly, we did not find P. bliccae on any endemic cyprinoids in Iraq or Iran, although some potential host species (e.g. C. macrostomum) were examined in our study. This observation might be influenced by some ecological factors of water environment such are temperature, pH and salinity (i.e. Shah et al., Reference Shah, Yousuf, Chishti and Ahmad2013; Gilbert and Avenant-Oldewage, Reference Gilbert and Avenant-Oldewage2016a, Reference Gilbert and Avenant-Oldewage2016b; Mbokane et al., Reference Mbokane, Theron and Luus-Powell2019; Aydoğdu et al., Reference Aydoğdu, Avenant-Oldewage, Dos Santos and Aydoğdu2020). In Turkey, P. bliccae was previously reported on S. fellowesii and Pseudophoxinus burduricus by İnnal et al. (Reference İnnal, Ünal, Çaglan, Civáňová and Özmen2020), and on Blicca bjoerkna, Scardinius erythrophthalmus, Abramis brama and V. vimba from many rivers flowing into the Baltic, Black and Caspian seas (i.e. Moravec, Reference Moravec2001; Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009).
On the basis of the obtained results, the host range of P. bliccae has expanded by six new host species. The third diplozoid species reported from a wide range of Middle East cyprinoids was P. homoion. Among Diplozoidae, this species has the widest range of host species concerning European cyprinoids (Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021) and in the Middle East (all main watersheds, i.e. Euphrates and Tigris basins, Namak basin, Aegean Sea basin and Caspian Sea basin), we recorded P. homoion on several new hosts of the genera Capoeta, Alburnus, Alburnoides and Barbus (Table 1). Other previously reported diplozoid species from the Middle East, i.e. Paradiplozoon chazarikum (Mikailov, 1973), and Paradiplozoon tadzhikistanicum (Gavrilov & Dzhaliliov, 1965) from Iran (Pazooki and Masoumian, Reference Pazooki and Masoumian2012), E. nipponicum, Paradiplozoon amurense and Paradiplozoon barbi from Iraq (Mhaisen and Abdul-Ameer, Reference Mhaisen and Abdul-Ameer2014), and P. megan, P. barbi and D. paradoxum from Turkey (Öktener, Reference Öktener2014) were not found on the cyprinoid species examined in our study, despite our investigation of the same reported catchments or the same host species for the aforementioned diplozoid species. Finally, our study revealed the presence of P. koubkovae n. sp. recorded for the first time on C. capoeta from Turkey and L. capito from Iran (both localities in the Caspian Sea basin).
Morphological intraspecific variability in monogeneans has been highlighted by several studies in relation to water temperature, host body size and/or geographical distribution (e.g. Ergens, Reference Ergens1976; Ergens and Gelnar, Reference Ergens and Gelnar1985; Littlewood et al., Reference Littlewood, Rohde and Clough1997; Kaci-Chaouch et al., Reference Kaci-Chaouch, Verneau and Desdevises2008; Kmentová et al., Reference Kmentová, Van Steenberge, Raeymaekers, Koblmüller, Hablützel, Bukinga, N'sibula, Mulungula, Nzigidahera and Ntakimazi2018; Rahmouni et al., Reference Rahmouni, Van Steenberge, Vanhove and Šimková2021, Reference Rahmouni, Vanhove, Šimková and Van Steenberge2022). Positive correlations between host body size and parasite body size or clamp size were previously reported also in diplozoids (Matějusová et al., Reference Matějusová, Koubková, Gelnar and Cunningham2002). Nevertheless, differences in the shapes of clamps have previously been used to differentiate between diplozoid species (Khotenovsky, Reference Khotenovsky1985b; Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009, Nishihira and Urabe, Reference Nishihira and Urabe2020). For instance, Nishihira and Urabe (Reference Nishihira and Urabe2020) utilized the size of the clamps as one of the key features to distinguish between E. nipponicum and Eudiplozoon kamegaii, although this feature is widely discussed by other authors as taxonomically unimportant (Matějusová et al., Reference Matějusová, Koubková, D'amelio and Cunningham2001b, Reference Matějusová, Koubková, Gelnar and Cunningham2002, Reference Matějusová, Koubková and Cunningham2004; Přikrylová et al., Reference Přikrylová, Mašová, Gelnar, Matla, Tavakol and Luus-Powell2018; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021). In the present study, we used all commonly used measurements of all the haptoral elements, and by removing the effect of host size we evidenced the morphological variability in the clamps of P. bliccae, indicating the morphological, and also genetic, differentiation on an interpopulation scale. PCA revealing two variants (A1 and A2) of P. bliccae in our study indicates the importance of using the measurements of all haptoral elements in morphological analysis. Considering host phylogeny, the distribution of the two P. bliccae variants (A1 and A2) appears to be unrelated to the phylogenetic relationships between the respective host species; however, their diversification seems to be related to the geographical distribution of their host species, i.e. we can assume the vicariant origin of P. bliccae variants. While P. bliccae specimens from variant A1 parasitized cyprinoids (S. fellowesii, L. kottelati, C. aydinensis, P. ninae, V. mirabilis and B. xanthos) from the Aegean Sea basin, the specimens from variant A2 parasitized cyprinoids (V. vimba) from the Black Sea basin.
Previous molecular studies suggested that even geographically distant populations of diplozoid species, more specifically those of P. homoion and E. nipponicum, exhibit no intraspecific variability in ITS2 (Matějusová et al., Reference Matějusová, Koubková, D'amelio and Cunningham2001b; Dos Santos and Avenant-Oldewage, Reference Dos Santos and Avenant-Oldewage2020). However, Benovics et al. (Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021) reported genetic intraspecific variability for Paradiplozoon ibericus from different cyprinoid species in the Iberian Peninsula (p-distance: up to 0.7%) i.e. intraspecific variability was reported in separate parts of this peninsula, and for P. megan (p-distance: up to 0.2%) from host species in geographically distant regions (i.e. Apennine and Balkan peninsulas). Intraspecific variability in the ITS1 and/or ITS2 regions was also documented for other monogeneans, such as Gyrodactylus von Nordmann, 1832 species (Matějusová et al., Reference Matějusová, Gelnar, McBeath, Collins and Cunningham2001a; Nitta and Nagasawa, Reference Nitta and Nagasawa2018), or Dactylogyrus Diesing, 1850 species (Šimková et al., Reference Šimková, Morand, Jobet, Gelnar and Verneau2004, Reference Šimková, Pečínková, Řehulková, Vyskočilová and Ondračková2007; Řehulková et al., Reference Řehulková, Rahmouni, Pariselle and Šimková2021) and thus is also expected in diplozoids. Nishihira and Urabe (Reference Nishihira and Urabe2020) used cytochrome c oxidase subunit I (COI) and ITS2 to reveal differences between two Eudiplozoon species (i.e. E. nipponicum and E. kamegaii). They suggested that the morphological and molecular divergence between the two species is the result of long-time adaptation to the specific host; thus, E. nipponicum on C. carpio should be reclassified as E. kamegaii, and E. nipponicum is strictly host-specific to Carassius spp. They documented significant intraspecific variability in COI (i.e. p-distance: 2.6–6.1% for E. kamegaii) and minor genetic variability in ITS2 (i.e. p-distance: 0–0.3% for E. kamegaii). The genetic distance found in our study (P. bliccae: p-distance: 0–0.8%) is greater than that shown for E. kamegaii by Nishihira and Urabe (Reference Nishihira and Urabe2020). A minor intraspecific genetic variability in ITS2 of P. bliccae was reported also by Unal et al. (Reference Unal, Innal, Civáňová, Stavrescu-Bedivan, Koubková, Ozmen and Gelnar2017); however, the authors did not provide complete sequence data, not p-distances, therefore, it is difficult to assess the level of variability. In general, despite the intraspecific genetic variability documented in diplozoids (e.g. Unal et al., Reference Unal, Innal, Civáňová, Stavrescu-Bedivan, Koubková, Ozmen and Gelnar2017; Nishihira and Urabe, Reference Nishihira and Urabe2020; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021), until now, there have been no studies to explore genetic intraspecific variability alongside morphological intraspecific variability.
The idea of the strict host specificity of Diplozoidae species was suggested by Sterba (Reference Sterba1957) and further promoted by Bychowsky and Nagibina (Reference Bychowsky and Nagibina1959). However, recent studies have reported several diplozoid species from a wide range of host species in various regions, e.g. Paradiplozoon hemiculteri from seven host species in East Asia (Dos Santos and Avenant-Oldewage, Reference Dos Santos and Avenant-Oldewage2020); P. megan from five host species in Europe; and P. ibericus from seven host species in Iberian Peninsula (Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021), which indicates that host specificity in diplozoids is often underexplored and should be interpreted carefully. Additionally, Sicard et al. (Reference Sicard, Desmarais and Lambert2001) suggested that host specificity may vary in different diplozoid species, as some species may infect only congeneric host species (i.e. one parasite species infects several host species of the same host genus) or phylogenetically closely related host species at a higher taxonomical level (i.e. host species belonging to the same family), while others infect a wide range of unrelated species. Paradiplozoon bingolensis is an example of a diplozoid species infecting the phylogenetically related species of one family i.e. Cyprinidae, and is currently geographically restricted to the Middle East. Benovics et al. (Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021) even proposed that some diplozoid species exhibit strong geographical specificity, e.g. P. ibericus is restricted only to the host species in the Iberian Peninsula and P. helleni is limited only to the few endemic species in the Balkans. In the current study, an endemic variant of P. bliccae (variant A1, see above) was documented, potentially suggesting an early speciation process in a small geographical region (two separate, yet adjacent, basins), which ultimately could have end in the emergence of new endemic Paradiplozoon species in the geographically isolated region.
The host specificity of diplozoids is often considered by some authors as a taxonomically important characteristic (Jiang et al., Reference Jiang, Wu and Wang1985; Al-Nasiri and Balbuena, Reference Al-Nasiri and Balbuena2016). As an example, diplozoid specimens recorded in a new region and/or on a new host species are often considered as a new species; e.g. previously described as Paradiplozoon parabramisi, Paradiplozoon diplophyllorchidis (Jiang et al., Reference Jiang, Wu and Wang1985), Paradiplozoon parapeleci (Jiang et al., Reference Jiang, Wu and Wang1985) and Paradiplozoon jiangxiense (Jiang et al., Reference Jiang, Wu and Wang1985), which were all revised as synonyms of P. hemiculteri (Jirsová et al., Reference Jirsová, Ding, Civáňová, Jirounková, Ilgová, Koubková, Kašný and Gelnar2018). All four ‘species’ were collected from the same region (Pearl River basins, China) and each of them parasitized different host species (Dos Santos and Avenant-Oldewage, Reference Dos Santos and Avenant-Oldewage2020). In the Middle East in 2016, P. iraqensis was described on C. macrostomum from Iraq (Al-Nasiri and Balbuena, Reference Al-Nasiri and Balbuena2016). This Paradiplozoon species shares many similar characteristics (e.g. clamp shapes, body shape, sickle size and handle size) with P. bingolensis described earlier on G. rufa from Turkey (Civáňová et al., Reference Civáňová, Koyun and Koubková2013). In the present study, Paradiplozoon specimens from C. macrostomum were found to be morphologically similar to P. iraqensis collected in the same area with some minor morphological differences (e.g. clamp size); however, the molecular data revealed that the collected specimens are genetically identical to P. bingolensis. Civáňová et al. (Reference Civáňová, Koyun and Koubková2013) suggested that the posterior jaw of P. bingolensis, which is not divided into medial and lateral sclerites, represents an important taxonomical feature for identification of this species (i.e. distinguishing P. bingolensis from the other congeners). The same morphological feature was evidenced also for P. iraqensis (Al-Nasiri and Balbuena, Reference Al-Nasiri and Balbuena2016) and we can assume that P. iraqensis is a morphological variant of P. bingolensis (i.e. it should be considered as synonym species). Unfortunately, Al-Nasiri and Balbuena (Reference Al-Nasiri and Balbuena2016) did not provide any genetic data for P. iraqensis, even though the molecular markers for the characterization and identification of diplozoids have been available from the early 2000s (Matějusová et al., Reference Matějusová, Koubková, D'amelio and Cunningham2001b, Reference Matějusová, Koubková, Gelnar and Cunningham2002, Reference Matějusová, Koubková and Cunningham2004; Přikrylová et al., Reference Přikrylová, Mašová, Gelnar, Matla, Tavakol and Luus-Powell2018) and various authors have suggested their inclusion in all taxonomical works (e.g. Jirsová et al., Reference Jirsová, Ding, Civáňová, Jirounková, Ilgová, Koubková, Kašný and Gelnar2018; Dos Santos and Aventnat-Oldewage, Reference Dos Santos and Avenant-Oldewage2020; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021).
Concerning the molecular phylogenetic reconstruction of diplozoids, previous studies revealed a polyphyly in Paradiplozoon (Matějusová et al., Reference Matějusová, Koubková and Cunningham2004; Civáňová et al., Reference Civáňová, Koyun and Koubková2013; Jirsová et al., Reference Jirsová, Ding, Civáňová, Jirounková, Ilgová, Koubková, Kašný and Gelnar2018; Přikrylová et al., Reference Přikrylová, Mašová, Gelnar, Matla, Tavakol and Luus-Powell2018; Dos Santos and Avenant-Oldewage, Reference Dos Santos and Avenant-Oldewage2020; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021), which was also revealed in our study. What is noteworthy is the nested position of A. polycotyleus and D. paradoxum within Paradiplozoon species, and the phylogenetic position of Sindiplozoon spp., Eudiplozoon spp. and Inustiatus spp. within Diplozoidae. Our ML and BI phylogenetic reconstructions indicated that diplozoids are divided into two major groups (clades A–D in one group and clades E and F in the other group), in line with previous studies by Dos Santos and Avenant-Oldewage (Reference Dos Santos and Avenant-Oldewage2020) and Benovics et al. (Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021). For interpreting the phylogenetic reconstruction, the geographical region of the current distribution of each diplozoid species should be taken into account. African species were included in clades A and E, which reveals the paraphyly of African diplozoid species previously also reported by Dos Santos and Avenant-Oldewage (Reference Dos Santos and Avenant-Oldewage2016) and Přikrylová et al. (Reference Přikrylová, Mašová, Gelnar, Matla, Tavakol and Luus-Powell2018), indicating that African diplozoid species had most likely two different origins. Benovics et al. (Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021) hypothesized that the Paradiplozoon species of clades E and F most likely dispersed through the Middle East and diversified in Africa or Southeast Asia, respectively, and that species of clade A (in their study, they divided clade A into two clades based on geographical distribution) diversified in Eurasia. Our results showed a larger clade encompassing both Eurasian and African species; however, it is still in line with that of Benovics et al. (Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021), as the phylogenetic relationships among the subclades are not fully resolved. Moreover, adding P. koubkovae n. sp. in the phylogenetic reconstruction revealed the role of the Middle East as a crossroad for cyprinoid hosts as P. ichthyoxanthon and P. vaalense branched out in Africa and P. koubkovae n. sp. has the sister position to them. It should be noted that using additional markers can further contribute to resolving the taxonomy of the Diplozoidae (Dos Santos and Avenant-Oldewage, Reference Dos Santos and Avenant-Oldewage2020; Nishihira and Urabe, Reference Nishihira and Urabe2020; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021). Moreover, addition of missing DNA sequences of other diplozoid species may help to resolve ambiguities in the phylogenetic relationships within the Diplozoidae. As for instance, inclusion of the P. moroccoensis sequence into the phylogenetic analysis revealed a topology slightly contradicting that presented by Dos Santos and Avenant-Oldewage (Reference Dos Santos and Avenant-Oldewage2020).
Finally, Dos Santos and Avenant-Oldewage (Reference Dos Santos and Avenant-Oldewage2020) suggested the need for major revision of the Diplozoidae. Especially, due to the phylogenetic position of Afrodiplozoon, they proposed re-evaluation of the status of two subfamilies of diplozoids. Concurrently with the phylogenetic relationships reported in our study, the previous authors (Dos Santos and Avenant-Oldewage, Reference Dos Santos and Avenant-Oldewage2020) also suggested all the species included in clade A represent a monophyletic and divergent taxon to other diplozoid genera, and the species within clade A should be taxonomically reclassified as Diplozoon due to consistent grouping of D. paradoxum with Paradiplozoon spp. This proposal is further supported by the morphology of the clamps in diplozoid species. Species included in clade A have six major parts in the anterior and posterior median sclerite of the clamps, the only exception being P. ichthyoxanthon with three parts. Sindiplozoon spp. (clade B) and Eudiplozoon spp. (clade C) have four parts in the anterior and posterior median sclerite of the clamps, but in different arrangements, while Inustiatus (clade D) has five major parts. Species of Paradiplozoon in clade F have two parts in the anterior median sclerite of the clamps and different arrangements in the posterior median sclerite to clade E (Pugachev et al., Reference Pugachev, Gerasev, Gussev, Ergens and Khotenowsky2009; Civáňová et al., Reference Civáňová, Koyun and Koubková2013; Avenant-Oldewage et al., Reference Avenant-Oldewage, Le Roux, Mashego and Van Vuuren2014; Dos Santos et al., Reference Dos Santos, Van Vuuren and Avenant-Oldewage2015; Jirsová et al., Reference Jirsová, Ding, Civáňová, Jirounková, Ilgová, Koubková, Kašný and Gelnar2018, Reference Jirsová, Koubková, Jirounková, Vorel, Zhou, Ding, Gelnar and Kašný2021; Přikrylová et al., Reference Přikrylová, Mašová, Gelnar, Matla, Tavakol and Luus-Powell2018; Benovics et al., Reference Benovics, Koubková, Civáňová, Rahmouni, Čermáková and Šimková2021; Cao et al., Reference Cao, Fu, Zou, Li, Wu, Wang, Blazhekovikj-Dimovska and Li2022). Considering the morphological similarities, the suggestion of Dos Santos and Avenant-Oldewage (Reference Dos Santos and Avenant-Oldewage2020) to separate the Paradiplozoon and Afrodiplozoon species in clade E into two genera needs to be taken with caution, as all the included species of Paradiplozoon (clade E) have the same clamp shape.
Conclusion
Recent knowledge on the diversity, phylogeny and ecology of Diplozoidae is fragmentary, and the resulting knowledge gaps are even more pronounced in the Middle East. This study was designed to fill some of these gaps and, at the same time, examine some issues relating to the current taxonomy of Diplozoidae. Our research suggests that the Middle East may represent the potential region of origin of different lineages of African species of Paradiplozoon. The intraspecific variability at the morphological and genetic levels in populations of P. bliccae from Middle Eastern cyprinoids suggests that the speciation process might have been promoted even in geographically small regions. In addition, we revealed a lower level of host specificity for currently known Paradiplozoon species. Overall, our study highlights the need for taxonomical re-evaluation within the Diplozoidae, based on an integrative morphological, ecological and molecular approach.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182023000446.
Data availability
All new DNA sequences of Paradiplozoon parasites and host species obtained during this study were deposite ddirectly into NCBI GenBank under accession numbers OP588752–OP588794 and OQ797981-OQ798023 respectively. Type material of new Paradiplozoon species was deposited in IPCAS under voucher numbers (IPCAS M-300 and IPCAS M-773).
Acknowledgements
We are grateful to Kateřina Francová, Robert Míč, Chahrazed Rahmouni and Jiří Vorel (Masaryk University, Brno, Czech Republic), Zeynep Zehra İpek (Recep Tayyip Erdogan University, Rize, Turkey) and Daniel Jablonski (Comenius University, Bratislava, Slovak Republic) for their help with the fish examination and parasite collection. We are also indebted to Hussein Valikhani and Amir Shahinpour (Shahid Beheshti University, Tehran, Iran) for obtaining necessary permits and fish collection in the field. Further thanks go to Mohammed Azeez Saeed and we acknowledge Salahaddin University in Erbil for providing sampling permits in Iraq and opportunity to conduct field collection. We kindly thank Matthew Nicholls for English revision of the final draft.
Author's contribution
F. N., M. B. and A. Š. conceived and designed the study. F. N., M. B., J. V., R. Š., A. S. T., C. K., S. A. and A. A. were responsible for collecting host specimens. J. V. and R. Š. confirmed the field host identification by DNA analyses. F. N., M. B. and E. Ř. were responsible for collecting parasitological materials and identification of the parasite specimens. F. N. and A. Š. performed statistical analyses. F. N., M. B., E. Ř. and A. Š. wrote the article. All authors approved the final manuscript.
Financial support
This study was funded by the Czech Science Foundation, project no. GA20-13539S.
Conflict of interest
There is no conflict of interest.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed. This study was approved by the Animal Care and Use Committee of the Faculty of Science, Masaryk University in Brno (Czech Republic).