Published online by Cambridge University Press: 27 September 2022
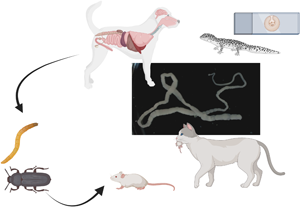
Although Joyeuxiella pasqualei is frequently detected in cats from Mediterranean Europe, information on its biology is still scarce. This cestode is relatively less frequently reported in dogs, possibly because it is often misdiagnosed with the better-known Dipylidium caninum. The occurrence of J. pasqualei proglottids in a dog living in a closed environment triggered us to delve into the biology of this cestode by collecting biological samples from lizards and a road-killed cat. Two reptile species, Podarcis siculus (Lacertidae), and Tarentola mauritanica (Geckonidae) were also collected in the garden and its surroundings. In addition, experimental infections with eggs obtained from gravid proglottids were performed in laboratory mice, and Tenebrio molitor (Coleoptera: Tenebrionidae) beetles. Proglottids from the dog's feces and adult cestodes detected at necroscopy of a cat were morphologically identified as J. pasqualei. Two out of 13 T. mauritanica collected in the garden had natural infections of J. pasqualei cysts in the liver and attached to the intestine. All P. siculus lizards and experimentally infected rodents and beetles were negative. DNA sequences obtained from J. pasqualei showed the highest nucleotide similarities with Versteria sp., Echinococcus sp., Raillietina sonini, Taenia polyacantha and D. caninum. Data herein provided show the inability of rodents to become infected by direct ingestion of gravid proglottids, suggesting a need for an invertebrate first intermediate host in the life cycle. Thus, more research study is advocated to better understand the biology of J. pasqualei such as its first intermediate host and its mechanism of transmission in reptiles and rodents.