Introduction
Clonorchis sinensis is the causative pathogen of human clonorchiasis. Humans are typically infected with the zoonotic trematode parasite upon consuming raw or undercooked freshwater fish carrying metacercariae. Clonorchiasis is mainly prevalent in East Asian countries, including Korea, China and northern Vietnam, and approximately 15–20 million individuals are estimated to be infected by the fluke globally (Lun et al., Reference Lun, Gasser, Lai, Li, Zhu, Yu and Fang2005; Qian et al., Reference Qian, Utzinger, Keiser and Zhou2016; Na et al., Reference Na, Pak and Hong2020). The main cause of the endemicity of clonorchiasis is the traditional habit of consuming raw or fermented freshwater fish, which is a significant hurdle in controlling and eliminating the disease in endemic countries.
Clonorchiasis is likely prevalent in Korea during the past decades. Control or elimination of C. sinensis infection has been attempted in Korea since 1970s. As a result, the overall prevalence of clonorchiasis in Korea has decreased in recent years; however, it still remains endemic to 5 major river basins and remains a non-negligible parasitic disease affecting human health in Korea. Here, we briefly review the epidemiology of C. sinensis and clonorchiasis in humans and intermediate hosts in Korea to provide insights into the current epidemiology of the parasite and the disease in Korea.
Life cycle of C. sinensis and its clinical manifestations
C. sinensis eggs are ingested by the freshwater snail, the first intermediate host. The eggs are hatched in its gastrointestinal tract and develop into miracidium. Miracidium proliferates into hundreds of cercariae in the snail, which are then released into the water. The actively swimming cercariae penetrate the second intermediate hosts, freshwater fish, develop into metacercariae in the muscles of freshwater fish, and are subsequently transmitted to definite mammalian hosts (Lai et al., Reference Lai, Hong, Su, Liang, Hide, Zhang, Yu and Lun2016).
Among freshwater snails, Parafossarulus manchuricus has been recognized as the major first intermediate host in Korea (Choi, Reference Choi1984). Snail species, such as P. anomalospiralis, Alocinma longicornis, Bithynia fushiana, B. misella, Melanoides tuberculata, Assiminea lutea and Thiara granifera, may act as the first intermediate hosts (Lun et al., Reference Lun, Gasser, Lai, Li, Zhu, Yu and Fang2005). Snails typically inhabit water bodies with slow flow or stagnant water in moist areas rich in organic sediment and aquatic vegetation.
Humans are the most important definitive hosts of C. sinensis. The known reservoir hosts include dogs, cats, rats, pigs, buffaloes, weasels and foxes (Na et al., Reference Na, Pak and Hong2020). Several laboratory animals, including rabbits, guinea pigs, hamsters, gerbils and mice, are reportedly susceptible to infection with C. sinensis metacercariae (Rim, Reference Rim1986; Lun et al., Reference Lun, Gasser, Lai, Li, Zhu, Yu and Fang2005; Tang et al., Reference Tang, Huang and Yu2016). Reservoir hosts serve as carriers of C. sinensis eggs and contaminate water sources.
The ingested metacercariae excyst in the duodenum of the human host, and the newly excysted juveniles rapidly migrate to the intrahepatic bile duct via the ampulla of Vater and the common bile duct and develop into adult worms (Kim et al., Reference Kim, Yoo, Kwak, Seok and Hong2011). Adult worms can survive for long periods (~20–30 years) in the bile ducts. During this time, infected individuals manifest diverse clinical symptoms of clonorchiasis resulting from continuous mechanical irritations of worms or chemical stimulations of excretory–secretory products (ESPs) (Na et al., Reference Na, Pak and Hong2020). The severity of symptoms varies depending on the worm burden, infection period and re-infection frequency. Common clinical manifestations at the initial stage include mild fever, diarrhoea, abdominal discomfort, malaise and anorexia. In serious and chronic infections, jaundice, biliary inflammation and bile duct obstruction are observed, resulting in cholangitis, cholelithiasis and cholangiectasis (Lun et al., Reference Lun, Gasser, Lai, Li, Zhu, Yu and Fang2005; Na et al., Reference Na, Pak and Hong2020). The parasite can also induce irreversible pathological changes or damage in the hepatobiliary tract. Hence, C. sinensis has also been recognized as a biological carcinogen that causes cholangiocarcinoma (CCA) by the International Agency for Research on Cancer (IARC) (Bouvard et al., Reference Bouvard, Baan, Straif, Grosse, Secretan, El Ghissassi, Benbrahim-Tallaa, Guha, Freeman, Galichet and Cogliano2009).
High prevalence of clonorchiasis in the 5 major river basins in Korea
C. sinensis, along with soil-transmitted helminths (STH), is likely prevalent in Korea for a long time as its eggs were discovered in ancient ruins and mummies (Zhan et al., Reference Zhan, Yeh, Shin, Chai, Seo and Mitchell2019; Oh et al., Reference Oh, Lee, Kim, Hong, Cha, Chai, Ha, Kang, Lim, Shin and Seo2021). Human helminthiasis was highly prevalent in Korea until the 1970s, when the per capita helminth parasite infection rate was estimated to be approximately 150% (Cho et al., Reference Cho, Chung and Lee2014a). The most prevalent helminth parasites were intestinal helminths, including STH, such as Ascaris lumbricoides, hookworms, and Trichuris trichiura and food-borne helminths, such as C. sinensis, Metagonimus yokogawai and tapeworms. To eliminate intestinal helminthic infections, the Korean Association for Parasite Eradication (KAPE; renamed the Korea Association for Health Promotion) was established in 1964. The Korean Government promulgated the ‘Parasitic Disease Prevention Act’ in 1966 (Lee, Reference Lee2007). In 1969, the first scientific report demonstrated that the national average positive rate for the presence of C. sinensis eggs was 4.7% (Table 1). However, a positive rate of 22% was observed in 40,581 residents of the 5 major river basins (Han-gang, Geum-gang, Seomjin-gang, Yeongsan-gang and Nakdong-gang; gang means river) with the highest positive rate of 48.1% in the Nakdong-gang area (Seo et al., Reference Seo, Rim, Loh, Lee, Cho, Park, Bae, Kim, Lee, Koo and Kim1969).
Table 1. Changing patterns of intestinal parasitic infections in nationwide surveys in Korea identified by the ‘Korea Intestinal Parasite Eradication Program’ conducted between 1969 and 2012

STH, soil-transmitted helminths; n.a., not available.
Source: Survey results were collected from the previous report ‘Prevalence of intestinal parasitic infections in Korea’.
Since the ‘Korea Intestinal Parasite Eradication Program’, which includes intensive health education and active screening of infected individuals followed by mass chemotherapy, began in 1971, the overall prevalence of STH infections has rapidly declined during the past decades in Korea (Cho et al., Reference Cho, Chung and Lee2014a) (Table 1). Based on the positivity rate of C. sinensis eggs in the examined population, the nationwide prevalence of C. sinensis infection was estimated to be 4.6% in 1971. Subsequently, the rate gradually decreased (Hong and Yong, Reference Hong and Yong2020).
Initiation of the Korea intestinal parasite eradication program
Nationwide application of praziquantel in 1983 led to a significant decrease in the prevalence of C. sinensis (Seo et al., Reference Seo, Lee, Chai and Hong1983). The declining prevalence of C. sinensis infections during the past decades may also be attributed to the strengthened health promotion and educational measures undertaken widely in endemic regions since 1973 (Lee, Reference Lee2007; Cho et al., Reference Cho, Chung and Lee2014a; Hong and Yong, Reference Hong and Yong2020). However, compared to the dramatic decline in other helminthic infections, especially STH infections, during the last few decades in Korea, the C. sinensis infection rate has remained a serious public health concern. Indigenous infections are still highly prevalent in the major river basins. In these regions, the prevalence is much higher than the national average infection rate. This suggests that C. sinensis is not distributed evenly throughout the country but is rather prevalent in endemic areas alongside the major river basins (Fig. 1). To finally eradicate fish-borne trematodes, including C. sinensis, and survey their second intermediate fish hosts, Korea Disease Control and Prevention Agency (KDCA; formerly known as Korea Centers for Disease Control and Prevention) launched the ‘Phase II Korea Intestinal Parasite Eradication Program’ in 2005 (Cho et al., Reference Cho, Shin, Lee and Park2016). Similar to the previous ‘Korea Intestinal Parasite Eradication Program’, this programme conducted a large-scale stool examination of inhabitants residing alongside the 5 major river basins in Korea (Cho et al., Reference Cho, Shin, Lee and Park2016; Ju et al., Reference Ju, Lee, Shin and Cho2017; Shin et al., Reference Shin, Lee, Bahk, Lee, Ju and Lee2020, Reference Shin, Bahk, Lee, Ju and Lee2021). The average positive rate of C. sinensis eggs was 8.7% over 10 years. These results demonstrated a high prevalence of 7.9–11.1% during 2005–2013. However, the prevalence was eventually reduced to 5.1% in 2014 (Table 2) (Cho et al., Reference Cho, Shin, Lee and Park2016). A recent nationwide survey of C. sinensis suggested that the estimated rate of C. sinensis infection has slightly decreased to 3.1% in 2019 and 3.8% in 2020 (Fig. 1). However, the rate is much higher among residents residing in Nakdong-gang, Seomjin-gang and Yeongsan-gang, suggesting that clonorchiasis has been a significant burden to health localities and households in the areas of the 3 river basins in Korea (Shin et al., Reference Shin, Lee, Bahk, Lee, Ju and Lee2020, Reference Shin, Bahk, Lee, Ju and Lee2021).
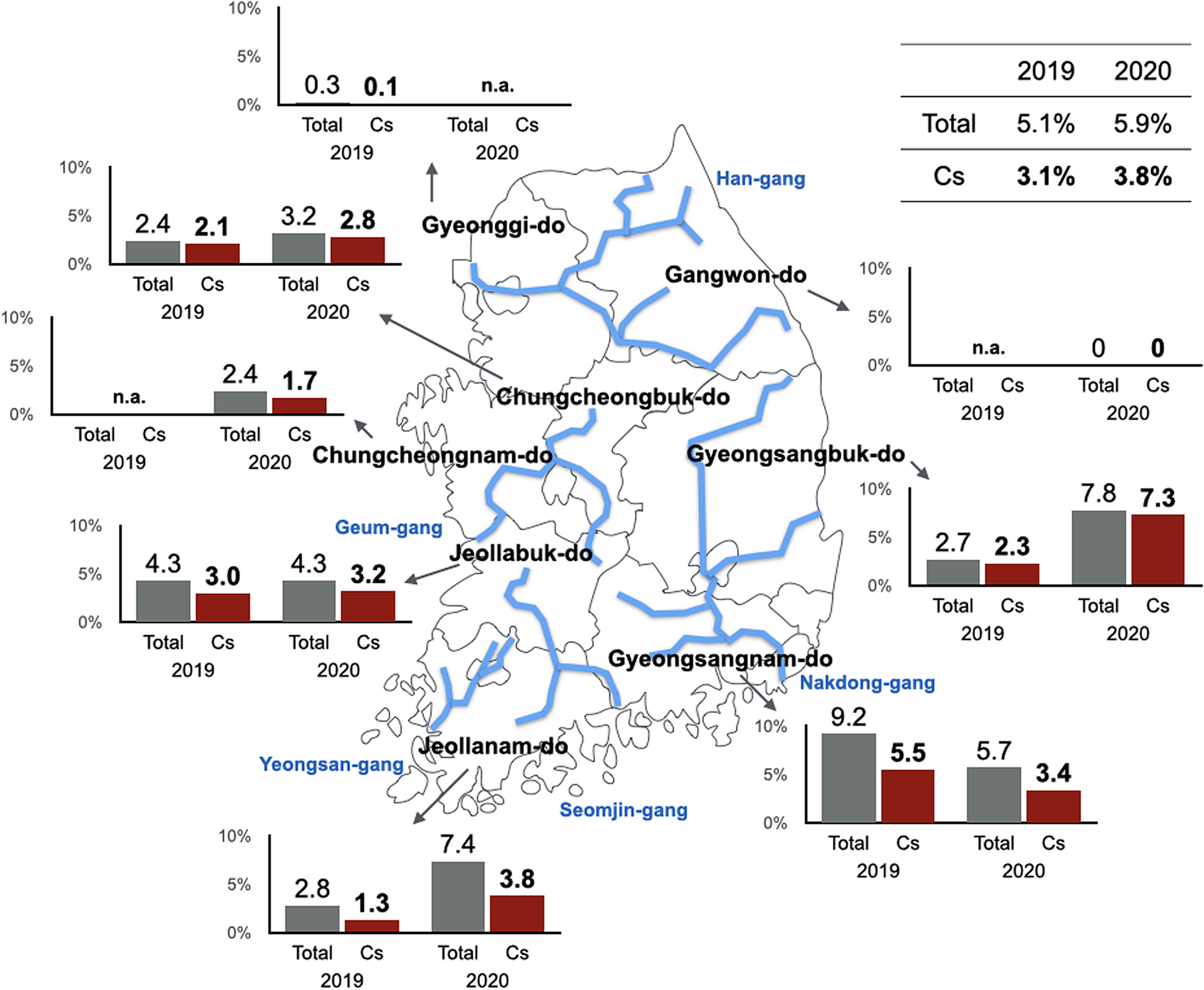
Fig. 1. Latest epidemic map of clonorchiasis in 8 administrative districts of Korea. The map was adapted from the ‘Phase II Korea Intestinal Parasite Eradication Program’ surveys conducted between 2019 and 2020 (Shin et al., Reference Shin, Lee, Bahk, Lee, Ju and Lee2020; Shin et al., Reference Shin, Bahk, Lee, Ju and Lee2021). Blue lines indicate major rivers in Korea. Cs, C. sinensis; Total, total positive rate of intestinal helminth eggs; do, province; n.a., not available.
Table 2. Changing trends in the endemicity of intestinal helminths in the major river basins identified by ‘Phase II Korea Intestinal Parasite Eradication Program’ between 2005 and 2014

n.a., not available.
Source: The survey results were collected from previous publications (Cho et al., Reference Cho, Shin, Lee and Park2016; Ju et al., Reference Ju, Lee, Shin, Kim, Lee, Kim, Bahk, Lee and Cho2018; Shin et al., Reference Shin, Lee, Bahk, Lee, Ju and Lee2020; Shin et al., Reference Shin, Bahk, Lee, Ju and Lee2021).
Epidemiological characteristics of clonorchiasis in Korea
Statistics examining the prevalence of clonorchiasis in Korea have revealed several epidemiological characteristics. A high positive rate indicating the presence of eggs is typically observed in the elderly population comprising individuals over 50 years old (Cho et al., Reference Cho, Lee, Lee, Cho, Cheun, Hong, Sohn and Kim2008) (Table 3), and re-infection after chemotherapy is also frequently observed in this population, suggesting that the old habit of eating raw freshwater fish from childhood is an important factor contributing to the high risk of C. sinensis infection (Rim, Reference Rim1990). Individuals are typically overconfident in the efficacy of praziquantel and ignore the risks of asymptomatic infection or re-infection (Rim et al., Reference Rim, Kim, Joo, Kim, Eom and Chung1996). Meanwhile, the infection rate in younger generations (<40 years old) is much lower than that in older generations. General changes in dietary patterns attributed to economic development and health promotion education for awareness of C. sinensis and clonorchiasis infections in schools may discourage younger individuals from eating raw freshwater fish. As clonorchiasis is closely associated with the socio-behavioural patterns of raw fish consumption, a higher prevalence of infection is observed in men than in women in all examined generations.
Table 3. Positive rates by age and survey years

Asterisk (*) indicate the highest positive rate per year; n.a., not available.
Source: The survey results were collected from the previous report ‘Prevalence of intestinal parasitic infections in Korea’ and publications (Cho et al., Reference Cho, Shin, Lee and Park2016; Ju et al., Reference Ju, Lee, Shin, Kim, Lee, Kim, Bahk, Lee and Cho2018; Shin et al., Reference Shin, Lee, Bahk, Lee, Ju and Lee2020; Shin et al., Reference Shin, Bahk, Lee, Ju and Lee2021).
C. sinensis as a causative factor of CCA
A significant correlation between the C. sinensis infection rate and CCA cases has also been reported in endemic areas, particularly in Gyeongsangnam-do (province), where Nakdong-gang flows (Choi et al., Reference Choi, Lim, Lee, Lee, Choi, Heo, Jang, Lee, Kim and Hong2006; Lim et al., Reference Lim, Ju, Franceschi, Oh, Kong, Hwang, Park, Cho, Sohn, Kim, Yoo, Hong and Shin2006; Shin et al., Reference Shin, Oh, Lim, Shin, Kong, Jung, Won, Park, Park and Hong2010). Among 185 outpatients with CCA, a significant correlation was observed between 167 (90.3%) patients and C. sinensis infection based on the radiological diagnosis of C. sinensis, history of eating raw freshwater fish and positive serological findings for C. sinensis (Choi et al., Reference Choi, Lim, Lee, Lee, Choi, Heo, Jang, Lee, Kim and Hong2006). To investigate the relationship between C. sinensis and CCA, the National Cancer Center (NCC) conducted an epidemiological survey (3169 residents; age range, 30–87 years) in 3 areas (Chuncheon, Chungju and Haman) with different CCA mortality rates. The highest prevalence of C. sinensis (31.3%) was detected in Haman, Gyeongsangnam-do, where the estimated CCA incidence rate was 5.5 per 100 000 individuals (Lim et al., Reference Lim, Ju, Franceschi, Oh, Kong, Hwang, Park, Cho, Sohn, Kim, Yoo, Hong and Shin2006). Eventually, the NCC and Ministry of Health and Welfare (MHW) officially announced that CCA is highly correlated with the high endemicity of C. sinensis in Korea (National Cancer Center, 2016).
Light and moderate C. sinensis infections function as impediments in controlling clonorchiasis
Despite the steady maintenance of the C. sinensis infection rate in Korea, the overall egg burden, determined by the number of eggs per gram of feces in patients with clonorchiasis, has decreased in recent years compared to that in past decades, except for 2 highly endemic areas, Nakdong-gang and Seomjin-gang basins (Shin et al., Reference Shin, Lee, Bahk, Lee, Ju and Lee2020). Low egg burden in patients implies mild infection or low worm burden. However, it is also an important hurdle for effective control and management of clonorchiasis. An accurate diagnosis followed by proper drug treatment of infected individuals is an efficient measure to obstruct the further transmission of C. sinensis infection. However, low egg burden has challenged the effective control of clonorchiasis. In common fecal examination, which is the gold standard diagnostic method for C. sinensis infection, patients with low egg burden can occasionally be determined to false negative (Korea Disease Control and Prevention Agency, 2009).
Several alternative diagnostic approaches to overcome weaknesses of traditional fecal examination method have been proposed. For immunological diagnosis, enzyme-linked immunosorbent assay is one of the most commonly used indirect serodiagnosis methods for C. sinensis infections. Soluble extracts or ESPs from adult worms have been employed as antigens to provide reliable diagnostic results (Na et al., Reference Na, Pak and Hong2020). However, such assays based on crude and ESP antigens are difficult to reproduce consistently and show cross-reactivity with infections caused by other trematodes. Many molecularly defined recombinant proteins have been suggested as useful antigens; however, they show moderate sensitivity and specificity. Examples include cysteine proteases (Na et al., Reference Na, Lee, Cho, Lee, Cho, Kho, Lee, Lee, Song, Park, Song and Kim2002; Kang et al., Reference Kang, Bahk, Cho, Hong, Kim, Sohn and Na2010), 7 kDa antigen (Lee et al., Reference Lee, Lee, Kim, Joo, Lee, Kim and Kim2002), glutathione S-transferases (Hong et al., Reference Hong, Yun Kim, Gan, Shen, Sukontason, Sukontason and Kang2002), paramyosin (Park et al., Reference Park, Kang, Na and Sohn2009; Kang et al., Reference Kang, Ju, Lee, Kim, Cho, Kim, Sohn and Na2015) and CsAg17 (Cho et al., Reference Cho, Lee, Kim, Song, Hong, Yoo, Tsuboi, Ha, Jung, Takeo, Han, Sripa, Hong, Chai, Nam, Pak and Kim2020). With respect to molecular characteristics, diagnosis can be made reliably by detecting C. sinensis nucleic acids in stool samples using polymerase chain reaction-based methods (Cho et al., Reference Cho, Na, Choi, Kim, Cho, Lee, Lim, Cha, Park, Pak, Lee, Hong and Kim2013). However, since nucleic acids need to be prepared from samples using specialized kits and devices, these requirements and high costs may be hurdle for local clinics and primary care institutions.
Freshwater fish as intermediate hosts
The reduction in the number of intermediate hosts due to natural environment destruction may have also contributed to the recent decline in C. sinensis prevalence. This suggests the importance of investigating the infection status of C. sinensis metacercariae in freshwater fish to survey and control intermediate hosts. To date, approximately 60 species of freshwater fish have been identified as second intermediate hosts of C. sinensis in Korea (Table 4) (Hong and Hong, Reference Hong and Hong2005; Kim et al., Reference Kim, Kim, Choi, Kim, Kim, Choi, Bae, Lee and Hong2008; Sohn, Reference Sohn2009, Reference Sohn2022). The infection rate of C. sinensis metacercariae in second intermediate hosts varies by season, region and freshwater fish species. Among the intermediate hosts of C. sinensis in Korea, several cyprinid fish species, such as Puntungia herzi, Pseudorasbora parva, Squalidus spp., Sarcocheilichthys spp. and Pseudogobio esocinus, are highly susceptible to C. sinensis infection. Recently, a total of 17 freshwater fish species, including Squalidus chankaensis, S. multimaculatus, Hemibarbus mylodon, Microphysogobio jeoni, M. longidorsalis, Ladislabia taczanowskii, Acheilognathus koreensis, A. majusculus, Acanthorhodeus macropterus, Rhodeus pseudosericeus, Zacco koreanus, Rhynchocypris oxycephalus, Odontobutis platycephala, Channa argus, Misgurnus anguillicaudatus, Micropterus salmoides and Lepomis macrochirus were newly identified as the second intermediate hosts for C. sinensis in Korea (Table 4) (Kim, Reference Kim1974; Cho et al., Reference Cho, Lee, Kim, Seok, Lee, Jeong, Na and Sohn2014b; Sohn et al., Reference Sohn, Na, Cho, Ju and Son2018, Reference Sohn, Na, Cho and Ju2019, Reference Sohn, Na, Cho, Ju, Kim, Hwang, No and Park2021a, Reference Sohn, Na, Cho, Kim, Hwang, No and Kim2021b, Reference Sohn, Na, Cho, Lee, Ju, Lee, Park and Ahn2021d). Notably, 2 exotic freshwater fish species, M. salmoides (large-mouth bass) and L. macrochirus (blue gill), were recently identified to harbour C. sinensis metacercariae (Sohn et al., Reference Sohn, Na, Cho, Lee, Ju, Lee, Lim, Son, Ko and Choi2021c). Both species were first introduced in Korea in the 1970s and have since spread to freshwater systems throughout the country. Further investigation on the mechanisms by which both freshwater fish species influence the infectivity of C. sinensis is necessary.
Table 4. Second intermediate hosts of C. sinensis in Korea

The infectivity of C. sinensis metacercariae in freshwater fish varies by region. It is generally low or moderate in freshwater fish in Han-gang and its streams (Cho et al., Reference Cho, Sohn, Na, Kim, Kong, Eom, Seok and Lee2011, Reference Cho, Lee, Kim, Seok, Lee, Jeong, Na and Sohn2014b; Sohn et al., Reference Sohn, Na, Cho, Lee, Choi and Seok2015) and Geum-gang (Cho et al., Reference Cho, Sohn, Na, Kim, Kong, Eom, Seok and Lee2011). Higher parasite infection rates have been found in freshwater fish obtained from Yeongsan-gang, Tamjin-gang and Seomjin-gang in Jeollanam-do (Cho et al., Reference Cho, Sohn, Na, Kim, Kong, Eom, Seok and Lee2011). Freshwater fish from Nakdong-gang in Gyeongsangbuk-do and Gyeongsangnam-do also showed high infection levels with C. sinensis metacercariae (Sohn et al., Reference Sohn, Na, Cho, Lee, Ju, Lee, Park and Ahn2021d, Reference Sohn, Na, Cho, Lee, Lee, Ju and Kim2021e) (Fig. 2). These patterns are highly correlated with the prevalence of human clonorchiasis.

Fig. 2. Infection rate of freshwater fish in the major river basins. The infection status was adapted from previous surveys as described in each reference. Rectangles indicate survey areas for freshwater fish collection. Red and black indicate positivity and negativity for C. sinensis eggs, respectively. Blue lines indicate major rivers in Korea. CsMs, C. sinensis metacercariae; cheon, stream; do, province; gun, county; n.a., not available.
Interdisciplinary One Health approach for eradication of C. sinensis
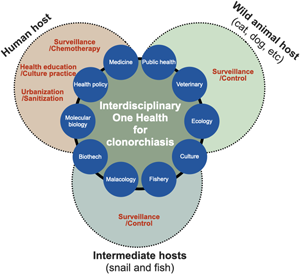
The control of C. sinensis and clonorchiasis has been successful in Korea. However, the control of the endemicity of C. sinensis is also necessary in some areas. The endemicity should probably be less than 1% for eradication. Recently, the One Health concept has been highly recommended for the integrated control of food-borne trematode infections by the World Health Organization expert consultation in Korea (WHO, 2017). One Health programme involves the collaboration of professionals across different disciplines aiming to adopt the best health measures for individuals, animals and the environment (Sripa et al., Reference Sripa, Tangkawattana and Sangnikul2017). Controlling zoonotic diseases requires interdisciplinary One Health approaches encompassing various sectors and disciplines, such as public health, veterinary medicine, clinical medicine, molecular biology, fisheries and malacology (Blake and Betson, 2017) (Fig. 3). Successful impact of the One Health approach has been demonstrated in the Lawa model, Khon Kaen, Thailand (Sripa et al., Reference Sripa, Tangkawattana and Sangnikul2017). The core strategy involved animal and human treatment, interventions based on influencing the ecosystem, food safety, hygiene and effective risk communication to accelerate and sustain the control of food-borne trematode infections.
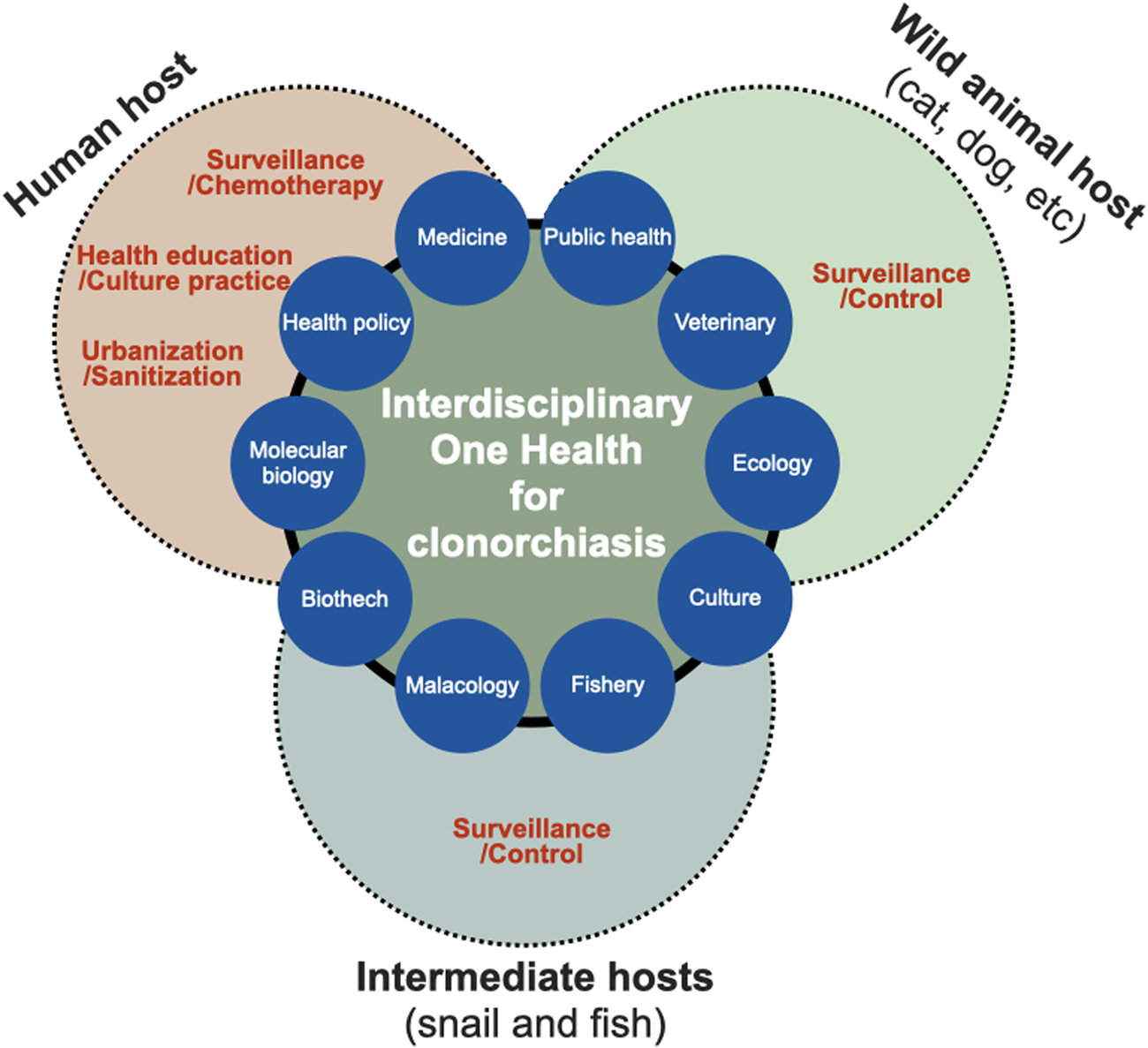
Fig. 3. Interdisciplinary One Health approach to accelerate effective control and eradication of C. sinensis infections. The One Health concept can be characterized as the approach involving the collaboration between professionals across different disciplines for the optimal health of individuals, animals and the environment.
First, wild and domestic animals in river areas may function as reservoirs or definite hosts of C. sinensis, and they are crucial factors in maintaining the life cycle of the parasite in the natural environment, contributing to the continuous endemicity of clonorchiasis (Min, Reference Min1981; Choe et al., Reference Choe, Jeong, Yang, Kim, Na, Lee, Park, Jeon and Eom2019). The nationwide positive rate of clonorchiasis was 1.4% in pigs, 2.4% in dogs and 1.9% in cats, whereas Gyeongsangnam-do showed the highest prevalence of 5.8, 6.1 and 4.5%, respectively (Min, Reference Min1981). Thus, veterinary surveillance should be performed to understand the spread of clonorchiasis from humans to animals and vice versa. KDCA operates Clo-Net to manage patient data with clonorchiasis and VectorNet to implement vector surveillance, although both are not yet open to the public at present. Therefore, it is necessary that the government extend the current surveillance system and open it for public purposes.
Second, the top priority is to end the cultural dietary habit of eating raw freshwater fish, which contributes to the persistent transmission of the fish-borne trematode to humans. The practice of consuming raw or undercooked freshwater fish has persisted for thousands of years in endemic regions (Oh et al., Reference Oh, Lee, Kim, Hong, Cha, Chai, Ha, Kang, Lim, Shin and Seo2021). Health promotion via informational programmes and education, such as publicity materials and educational videos (‘Do not eat raw freshwater fish’ URL: https://youtu.be/llqIwPhMcTU) among residents residing in the highly endemic river areas, should be strengthened to change their eating habits with cooperative efforts of the KDCA, local government and public health professionals.
Third, cases of light and moderate C. sinensis infections remain a challenge for accurate diagnosis owing to insufficient sensitivity and difficulty in identifying morphologically similar parasite species in fecal samples. Moreover, fecal examination method is unsuitable for large-scale surveys because of the time- and labour-intensive tasks involved inevitably. Thus, the development and application of simple and sensitive diagnostic methods to detect patients, especially low worm burden individuals, are urgently needed. In the era of artificial intelligence, deep-learning-based egg recognition may be an alternative method to differentiate C. sinensis eggs from the eggs of other intestinal trematodes (Jiménez et al., Reference Jiménez, Maya, Velásquez, Barrios, Pérez and Román2020). Collaboration between several disciplines and biotechnology to develop simple, easily adaptable on-site and reliable diagnostic methods may contribute to mass surveys (Ju et al., Reference Ju, Lee, Cho and Park2016; Yoo et al., Reference Yoo, Kang, Le, Pak, Hong, Sohn and Na2020).
Concluding remarks
Clonorchiasis is a preventable parasitic disease among individuals who do not consume raw freshwater fish. However, a non-negligible prevalence of the disease is still observed in Korea, especially among the inhabitants of the 5 major river basins. Case confirmation in the field is difficult, and confirmed cases are incompletely cured owing to treatment failure or re-infection. In the wake of the increasing number of mild infections, a health concept that considers an interdisciplinary approach is required to control and eliminate C. sinensis and clonorchiasis in Korea. Health promotion via increasing awareness and health education to prevent the consumption of raw freshwater fish among residents living in high-risk areas is also necessary.
Data availability
The original data in the present study are available from the corresponding author upon request.
Author contributions
WGY and BKN designed the study. WGY, WMS and BKN conducted data gathering. WGY, WMS and BKN wrote the article.
Financial support
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF2018R1C1B6005581) and Institute of Health Sciences of Gyeongsang National University (IHS GNU-2021-03).
Conflict of interest
None.
Ethical standards
None.