Introduction
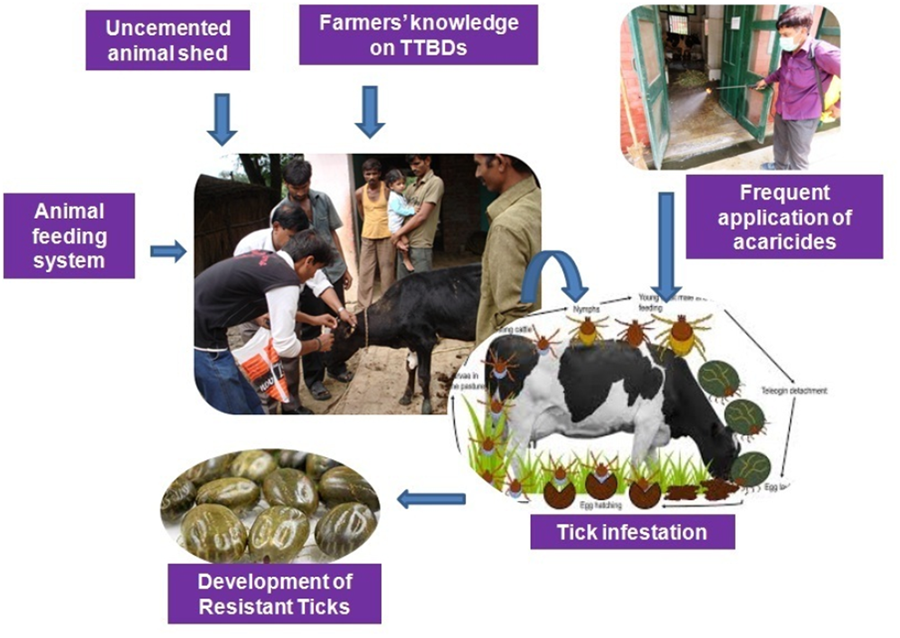
Tick infestation and its impact are significant challenges that limit livestock output in cattle-rearing communities mainly in tropical and subtropical countries. It is reported that about 80% of cattle population globally are adversely affected by ticks and the tick-borne diseases (TTBDs; de Castro et al., Reference de Castro, James, Minjauw, Di Giulio, Permin, Pegram, Chizyuka and Sinyangwe1997). In India, the key species of cattle ticks are Rhipicephalus microplus and Hyalomma anatolicum (Ghosh et al., Reference Ghosh, Azhahianambi and de la Fuente2006) which serve as a vector of fatal diseases like anaplasmosis, babesiosis and theileriosis. Globally, ticks cause economic annual losses of US$22–30 billion in cattle by transmitting tick-borne diseases (TBDs) (Hurtado and Giraldo-Rios et al., Reference Hurtado and Giraldo-Ríos2018). In Brazil, R. microplus alone causes a loss of $32.4 million per year (Grisi et al., Reference Grisi, Leite, Martins, Barros, Andreotti, Cançado, León, Pereira and Villela2014). However, losses estimated due to TTBDs varies by country such as $3.0 million (Graham and Hourrigan, Reference Graham and Hourrigan1977) in the USA, $573.16 million in Mexico, $168.0 million in Colombia (Rodríguez-Vivas et al., Reference Rodríguez-Vivas, Grisi, Ṕerez de Leon, Villela, Torres-Acosta, Fragoso Sanchez, Romero Salas, Rosario Cruz, Saldierna and García Carrasco2017), $250.0 million in Australia (Meat and Livestock Australia report 2020), $364.0 million in Tanzania (Kivaria, Reference Kivaria2006), $6.7 million in Puerto Rico and $5.0 million in Zambia (Senbill et al., Reference Senbill, Hazarika and Baruah2018). In India, economic impact of TTBDs was estimated over $787.63 million per annum (Singh et al., Reference Singh, Kumar, Sharma, Jacob, Verma, Singh, Shakya, Sankar and Ghosh2022).
No specific study was focused to create a more efficient, long-lasting and comprehensive tick control approach, or to assess the performance of existing control strategies beyond the traditional application of acaricides (Jongejan and Uilenberg, Reference Jongejan and Uilenberg2004). In India, generally, 4 chemical classes of acaricides, i.e. organophosphates, synthetic pyrethroids, amidines and avermectins (Fular et al., Reference Fular, Sharma, Upadhaya, Nandi, Nagar, Bisht, Shakya, Kumar, Kumar, Kumar and Ghosh2021) are commonly used for tick management. Some of these chemicals are not effectively working against ticks in many parts of the country due to the development of acaricidal resistance (Bisht et al., Reference Bisht, Kumar, Sharma, Nandi, Singh, Fular, Nagar and Ghosh2021; Shakya et al., Reference Shakya, Sharma, Kumar, Upadhaya, Nagar, Singh, Sankar and Ghosh2023). The use of acaricides on animals is prevented due to increase in resistant tick populations, their high cost, negative effects on unintended species and acaricidal residues in animal products (Singh et al., Reference Singh, Kumar, Sharma, Jacob, Verma, Singh, Shakya, Sankar and Ghosh2022). The success of tick control programme is based on comprehensive farmers' knowledge on TBDs, their perspective on efficient control methods and the socio-cultural environment in which the programme is carried out. The information is usually collected using the commonly used knowledge, attitude and practices (KAP) survey (Launiala, Reference Launiala2009). The method sets the initial standard for future evaluation and analysis of the impact of knowledge, attitude and practice on modifying TBD-related issues. It proposes an intervention approach that takes into account the unique local conditions and the cultural variables that shape them, and designs activities that are appropriate for the particular community concerned (Gumicio et al., Reference Gumicio, Merica, Luhman, Fauvel, Zompi and Ronsse2011). Despite criticism for generalized data of a large population for planning purposes, KAP surveys on TBDs have played a significant role in developing effective intervention strategies (Butler et al., Reference Butler, Sedghi, Petrini and Ahmadi2016; Zoldi et al., Reference Zöldi, Turunen, Lyytikäinen and Sane2017; Niesobecki et al., Reference Niesobecki, Hansen, Rutz, Mehta, Feldman, Meek, Niccolai, Hook and Hinckley2019; Gupta et al., Reference Gupta, Gupta and Kumar2021).
The animal owners of Dhar district of Madhya Pradesh face problem of resistance development in ticks and their management due to lack of KAP-based data. These data are essential to formulate suitable strategy to manage resistant ticks and to improve livestock health and the income of the marginal animal owners. Thus, to tackle the problem in the targeted region, a KAP-based study was conducted to assess the influence of TBDs on livestock productivity and determination of resistance status of tick populations, and the control strategies adopted by livestock owners. The collected data will aid in creating efficient animal health initiatives to boost livestock output and to enhance the socioeconomic status of livestock owners of targeted region.
Materials and methods
Study area
Dhar district is located in Malwa region of Madhya Pradesh of India and was selected for conducting the KAP survey. It possesses a diverse terrain with altitudes ranging from 150 to 600 m above sea level, influenced by the Vindhya Range. The vegetation consists of dry deciduous forests, including teak, sal and bamboo, with more dense forest cover in the hilly regions. The semi-arid climate and topographical variations contribute to the presence of grasslands and scrub forests in lower areas. The geographic locations of different sub-divisions of Dhar district are Dhar (DHA, 75.32°N, 22.61°E), Manawar (MAN, 75.08°N, 22.23°E), Sardarpur (SAR, 74.97°N, 22.65°E), Kukshi (KUK, 74.75°N, 22.20°E) and Gandhwani (GAN, 75.08°N, 22.23°E). Cattle and buffaloes are primarily reared for milk production, contributing significantly to the livelihoods of the local population. However, challenges such as limited access to quality feed, veterinary services and water resources can affect the productivity of milch animals in the region. The organized farms included more than minimum 10 milch animals and well-maintained shelter with proper cemented flooring infrastructure for animals. On the other hand, unorganized farm included household animals which had mud flooring and no proper amenities and only 2 or 3 animals were maintained for personal purpose.
Questionnaire survey
A systematic questionnaire was designed to gather data on several aspects associated with cattle productivity and TTBDs. A questionnaire proforma was designed in a multiple-choice form as per the guidelines (Thrusfield, Reference Thrusfield2018), with modifications made via both informal and formal testing processes. The questionnaire proforma contained several subjects like socio-demographic information, animal sheds, animal feeding methods, shed conditions, farming practices, methods of acaricidal application, risk factors, etc. The survey was carried out bi-monthly from February 2022 to January 2023 to monitor seasonal variations in cattle productivity and the prevalence of TBDs and the questionnaire was provided to livestock owners at the surveyed places. The study authors conducted the survey through face-to-face interviews with the owner. There were no specific inclusion and exclusion criteria for the participants in the study. The survey was intended for household heads; however, if there were other persons involved in livestock rearing in the household, they were asked to be in the survey as well. The questionnaire underwent pilot testing to ensure its effectiveness in gathering correct information (Williams, Reference Williams2003). Prior to providing the questionnaire to the targeted participants, it was reviewed by a number of experienced investigators of epidemiological study. Then, the data were carefully collected, analysed and screened for accuracy. The farmers selected for the investigation were chosen for their willingness to participate and operational convenience. They were owners of ruminant herds consisting of 5–15 animals (Hussain et al., Reference Hussain, Hussain, Ho, Li, George, Rehman, Zeb and Sparagano2021). About 200 individual interviews were conducted with livestock owners using a developed questionnaire.
Tick collection and processing
The biological samples of R. microplus ticks were collected from different regions of Dhar district following a randomized sampling procedure. Tick samples were collected from cattle and buffaloes of the households and well-managed dairy farms. Engorged female ticks were collected in labelled sample bottles covered with cotton cloth, and brought to the research centre. In the laboratory, at least 100–150 engorged female ticks collected from each sub-division were pooled and placed in Petri plates (5 ticks per plate) and maintained in the laboratory (Ghosh and Azhahianambi, Reference Ghosh and Azhahianambi2007). They were then kept at 28°C and 85 ± 5% relative humidity for normal oviposition. The ticks procured from Dhar, Manawar, Sardarpur, Kukshi and Gandhwani sub-divisions were encoded as DHA, MAN, SAR, KUK and GAN isolates, respectively. The eggs laid by the female ticks from each sub-division were combined and identified as a representative sample of that sub-division. The eggs of each sub-division were pooled, collected and stored in tick-rearing tubes. Once hatched, the larvae were placed in an incubator set at 28°C and 85 ± 5% relative humidity for 8–10 days for larval-based experiments.
Identification of ticks
The collected tick samples of both the sexes were observed morphologically under stereomicroscope. The specific characters of R. microplus and H. anatolicum were identified with the help of the book ‘Helminths, Arthropods and Protozoa of Domesticated Animals’ (Soulsby, Reference Soulsby1982) and then characterized them.
Reference tick
The reference susceptible IVRI-I strain of R. microplus was used as the reference tick for resistance characterization. The IVRI-I strain is already characterized as susceptible to most of the chemical acaricides in the Entomology laboratory of Indian Veterinary Research Institute, Izatnagar.
Chemical acaricides
Technical grade deltamethrin (DLM) and fipronil (FIP) were procured from Sigma Aldrich (St. Louis, MO, USA) and their stock solutions of 5000 and 1000 ppm, respectively, were prepared in methanol. Working concentrations of DLM (60, 90, 120, 150 and 180 ppm) and FIP (10, 15, 20, 25 and 30 ppm) were prepared in distilled water from their stock solutions and were tested for resistance characterization in collected tick samples.
Resistance characterization
Larval packet test (LPT)
A modified version of the larval packet test (LPT) as recommended by the FAO (2004) was used. The packets were prepared in triangular shape from Whatmann filter paper No. 1 measuring 5.5 cm × 5 cm. These packets were soaked with 0.7 mL solution of acaricide and then dried at 37°C in hot air oven. After drying, 1 side of the packets was sealed with adhesive tape. Then, about 150–200 larvae of 7–10 days old were introduced in the packets and sealed using a ‘bulldog’ clips. The packets were then kept in for 24 h in a biological oxygen demand (BOD) chamber at 28°C and 85 ± 5% relative humidity. After 24 h, these packets were removed from the BOD, and opened on white paper sheet under electric lamp to observe dead and alive larvae. The larvae only moving their legs were considered as dead while running larvae were counted as alive. Accordingly, the mortality percentage of larvae was determined by counting the number of alive and dead larvae. Three replications were maintained for each concentration of acaricides along with control with distilled water.
Larval immersion test (LIT)
Shaw (Reference Shaw1966) was the initial developer of the larval immersion test (LIT). The Shaw's immersion sandwich method involves larval immersion in an acaricide solution or suspension. For the assay, more than 300 larvae were transferred into 1.5 mL microcentrifuge tubes (3 repetitions per dilution) with the help of drawing brush and then an amount of 0.75 mL of working solution of the acaricide was poured in these tubes. The larvae were submerged for 10 min and agitated intermittently. After opening the tubes, approximately 100 larvae were transferred to filter paper packets and sealed with ‘bulldog’ clips. The packets were kept at 28°C and a relative humidity of 85 ± 5% for 24 h. Control groups of each acaricide were also immersed in distilled water in the same way. After 24 h, larval mortality was assessed as mentioned in LPT.
The resistance status of field isolates was determined on the basis of resistance ratio (RR). The resistance ratio (RR50) is the ratio of LC50 value of an acaricide for field ticks and LC50 value of the acaricide for reference susceptible IVRI-I strain (Castro-Janer et al., Reference Castro-Janer, Rifran, Piaggio, Gil, Miller and Schumaker2009). Ticks were then classified according to various resistance levels as per the method of Sharma et al. (Reference Sharma, Kumar, Kumar, Nagar, Singh, Rawat, Dhakad, Rawat and Ray2012).
Statistical analysis
The questionnaire data from 200 respondents were transferred to the Microsoft Excel 2010 sheet for proper management and analysis. The proportions of variables recorded in questionnaires were analysed following descriptive statistics (frequencies and percentages). The data were analysed by Epi Info™ software (Centers for Disease Control and Prevention, Atlanta, GA, USA). Association of socio-demographic characteristics to level of tick infestation was analysed by χ 2 test. Simple logistic regression analysis through R-software package (dplyr) was also performed to observe the association of respondent's knowledge and level of tick infestations (Wickham et al., Reference Wickham, François, Henry and Müller2021). The dose–response data of LPT and LIT were subjected to probit analysis (Finney, Reference Finney1971) using GraphPad Prism v.5 statistical software (GraphPad Software, San Diego, CA, USA) to determine LC50 values of each acaricide.
Results
Collection of ticks and farm management practices
The tick isolates were collected from the households and well-managed dairy farms located in 5 sub-divisions of Dhar district of Madhya Pradesh. There were both cross-bred and native breeds of cattle and buffaloes in the district. The surveyed animals were found to have a moderate (>50–100 ticksper animal) to high (>150–200 ticks per animal) level of tick infestations. Despite repeated applications of different synthetic acaricides such as cypermethrin, DLM, ivermectin and amitraz, a significant number of farmers reported the failure of tick control. During the survey, it was noticed that the application of FIP in the dairy farms is not frequent and almost lacking. However, the application schedule for other synthetic acaricides was not properly maintained and the animals were treated whenever tick infestation was visible on animals. The frequency of acaricidal treatment of household animals was comparatively lower than those maintained in well-managed dairy farms. The targeted area was highly dominated by the tribal population where animals were kept in small to big huts made of mud, concrete and thatched roofs with no proper acaricidal dose and application. It was noticed that the farmers rarely applied insecticides in the animal sheds to eradicate off the host tick stages (Table 1).
Table 1. Questionnaire data collected from surveyed places for determination of pattern of acaricidal application in fields

a Application frequency: frequent = 10–14 applications/tick active season; occasional = 4–8 application/tick active season.
Analysis of socio-demographic characteristics of the respondents
The study comprised 200 farmers including literate and illiterate from 5 sub-divisions of Dhar district and more than 90% famers belonged to rural areas. It was interesting to mention that farmers showed their interest to adopt new techniques and technologies for the management of TBDs. Face-to-face interviews revealed that about 40% (33.15–47.15) respondents were literate and 60% (52.85–66.85) were illiterate, out of which 55% (47.82–62.02) were using uncemented floors and 45% (37.98–52.18) were using cemented floors for their animals. Respondents adopted different feeding methods for their animals as observed during the survey. It was found that about 37.5% (31.25–45.11) respondents fed their animals in manger, only 5% (2.42–9.00) adopted grazing system and the rest (42.5%) adopted a mixed type of feeding system. Only 25% (19.16–31.60) respondents had knowledge about TTBDs while 75% (68.40–80.84) were not aware of it. During the study, we observed that the cypermethrin was preferred for animal application by the livestock owners [i.e. 35% (28.41–42.05)] followed by DLM [29% (22.82–35.82)] and ivermectin [15% (10.35–20.72)] while only 9% (5.42–13.85) farmers were applying amitraz for tick control. The respondents [12% (7.84–17.33)] were using more than 1 acaricide without maintaining any fixed application pattern and hence they were considered in the mixed category. A favourable attitude towards different tick control methods was shown by 36.5% (29.82–43.58) respondents in which manual hand picking as well as chemical control methods were the most preferred methods (Table 2).
Table 2. Socio-demographic characteristics of the respondents

Association of socio-demographic characteristics to level of tick infestation by χ 2 test
Analysis by χ 2 test revealed that the animals of literate respondents were significantly (P < 0.05) less susceptible to tick infestation as compared to those of illiterate respondents (P = 0.0180). The animals kept in the uncemented floor of shed exhibited a high intensity of tick infestation (0.0029). The animals having manger feeding were observed to be less susceptible to ticks as compared to grazing and mixed feeding animals (P < 0.0335) indicating the significant association (P < 0.05). Amongst 200 respondents, only 50 exhibited knowledge regarding TBDs. The data obtained were found to be statistically significant at a 5% level (P = 0.0063) (Table 3). Insignificant differences were observed between tick infestation level and acaricides used for tick control by respondents.
Table 3. Association between socio-demographic characteristics of the respondents (n = 200) and tick infestation

* Significant at P < 0.05 **Significant at P < 0.01
Association of socio-demographic characteristics to level of tick infestation by logistic regression analysis
The data analysed by logistic regression revealed that the animals of female livestock owners were 2.20 times (OR 95% CI 1.13–4.27) more likely than male livestock owners to experience tick infestation. Although the level of tick infestation was not considerably impacted by respondents' educational levels, their attitudes towards various tick-control strategies were greatly influenced. Similarly, the livestock owners without having knowledge of TBDs were 1.28 times (OR 95% CI 0.42–3.86) more likely to have a high level of tick infestation (Table 4). Moreover, the low-level infestation was recorded in the animals of respondents having a favourable attitude towards different tick control methods (OR 1.04, 95% CI 0.4–2.66). The respondents who practiced grazing as a sole method of feeding for their animals were likely to be more susceptible by 6 times (OR 95% CI 2.93–12.28) to ticks and had a heavy tick infestation as compared to mangers and mixed feeding practices. No significant difference between level of tick infestation and acaricides used for tick control was observed (Table 5).
Table 4. Simple logistic regression analysis for the estimation of the association between socio-demographic variables of respondents and binary outcome

Significant at P < 0.05; OR, odd ratio.
Table 5. Multiple logistic regression analysis for the estimation of the association between practices of respondents with level of tick infestation

Significant at P < 0.05
Association of socio-demographic characteristics to level of tick infestation by R software analysis
The multiple logistic regression analysis showed that shed floor type, feeding system and acaricides used for tick control were the significant variables in this model. Respondents having uncemented animal sheds were 5.16 times (OR 5.16) more likely to have a high level of tick infestation in their animals as compared to those having cemented floor of sheds. The respondents adopted 3 types of feeding systems: grazing, manger and mixed feeding. The respondents adopting a grazing system showed that their animals were 4.10 times (OR 4.10) more likely to have a high-level infestation as compared to those kept in a manger or mixed feeding system while the other variable in the model is held constant. The acaricides commonly used by respondents also significantly affected the tick infestation level (OR 1.77). The interaction term (i.e. sex, literacy, knowledge about TTBDs and attitude towards tick control) was not significant in this analysis (Fig. 1, Table 6).
Table 6. Association between socio-demographic variables of respondents and tick infestation level by R software analysis with different R packages

The odds ratio is the ‘exponential’ of the estimate obtained in glm model (log regression). Significant at ***P < 0.001, **P < 0.01, *P < 0.05.

Figure 1. Variable importance model for each contributing factor in tick infestation.
Resistance status of deltamethrin and fipronil
To identify the generation of acaricide resistance in tick population collected from different sites of a district, the larval-based assays, i.e. LPT and LIT, were conducted in the laboratory against DLM and FIP and the larval mortality was recorded after 24 h. In case of R. microplus, the tested isolates were highly resistant to DLM (RR = 33.9–39.9) as observed by LPT (Table 7). Surprisingly, in case of LIT, a low level of resistance against DLM was detected (RF = 1.2–4.3) (Table 7). All the isolates were susceptible to FIP by LPT (RR = 0.17–0.24) and LIT (RR = 0.48–0.51). The LC50 values were ranging from 400.69 to 471.6 ppm and 15.02 to 51.42 ppm against DLM in LPT and LIT format. The lower mortality slopes were observed in all isolates as compared to reference susceptible IVRI-I strain (3.42 ± 0.49) indicating the presence of more heterogeneous DLM-resistant population of R. microplus. The results indicated that the ticks of this area developed resistance against DLM (Fig. 2, Table 7).
Table 7. Mortality slope, R 2, LC50 with 95% CI and RR50 values of deltamethrin and fipronil against larvae of R. microplus by using LPT and LIT

DHA, Dhar; GAN, Gandhwani; KUK, Kukshi; MAN, Manawar; SAR, Sardarpur; IVRI-I, reference susceptible tick strain; RR50 (median), resistance ratio; RL, resistance level [susceptible (S) = RR < 1.4; level I: 1.5 < RR < 5; level II: 5.1 < RR < 25; level III: 26 < RR < 40; level IV: RR > 41].

Figure 2. Regression curves showing probit mortality in larval packet test (LPT, A and B) and larval immersion test (LIT, C and D) against log concentration of chemical acaricides (deltamethrin and fipronil) in 5 field isolates of Rhipicephalus microplus from Dhar district, Madhya Pradesh, India: DHR, Dhar; GAN, Gandhwani; KUK, Kukshi; MAN, Manawar; SAR, Sardarpur.
The LPT- and LIT-based resistance data of H. anatolicum against DLM and FIP are documented in Table 8. Results revealed that all the field isolates were resistant to DLM at level II (RF = 11.1–16.6) by LPT. Similarly, in case of LIT, an initiation of resistance to DLM was detected (RF = 1.5–2.3). Like R. microplus all the isolates of H. anatolicum were also found susceptible to FIP. The LC50 values were in the range from 132.17 to 194.90 ppm and 18.53 to 28.04 ppm against DLM in LPT and LIT, respectively. The lower mortality slopes were seen in all the samples in comparison to IVRI-I strain (3.42 ± 0.49) except Dhar isolate (5.002 ± 1.23) indicating the presence of more heterogeneous DLM-resistant populations of H. anatolicum (Fig. 3).
Table 8. Mortality slope, R 2, LC50 with 95% CI and RR50 values of deltamethrin and fipronil against larvae of H. anatolicum by using LPT and LIT

DHA, Dhar; GAN, Gandhwani; KUK, Kukshi; MAN, Manawar; SAR, Sardarpur; IVRI-I, reference susceptible tick strain; RR50 (median), resistance ratio; RL, resistance level [susceptible (S) = RR < 1.4; level I: 1.5 < RR < 5; level II: 5.1 < RR < 25; level III: 26 < RR < 40; level IV: RR > 41].

Figure 3. Regression curves showing probit mortality in larval packet test (LPT, A and B) and larval immersion test (LIT, C and D) against log concentration of chemical acaricides (deltamethrin and fipronil) in 5 field isolates of Hyalomma anatolicum from Dhar district, Madhya Pradesh, India: DHR, Dhar; GAN, Gandhwani; KUK, Kukshi; MAN, Manawar; SAR, Sardarpur.
Discussion
This research is the first to investigate the knowledge, attitudes and behaviours of livestock owners in Madhya Pradesh, India, about ticks and tick control measures. The report also covers the perceptions of stakeholders and livestock farmers in the study region about these limitations. Historically, most of the animal health researches worldwide were focused on pastoral regions (Catley et al., Reference Catley, Admassu, Bekele and Abebe2014; Queenan et al., Reference Queenan, Mangesho, Ole-Neselle, Karimuribo, Rweyemamu, Kock and Häsler2017). Pastoral communities predominate in the majority of African nations; in contrast, mixed crop-livestock farming practices and production systems are widespread in India (Hemme et al., Reference Hemme, Ndambi and Schröer-Merker2013). More than half of the surveyed respondents did not know how their livestock become infested with ticks or where ticks are typically located in the environment, despite the fact that every respondent had encountered a tick problem. According to some respondents, ticks are less prevalent in the winter. As temperature is an important factor in several tick developmental processes, including moulting, oviposition and questing, low temperature in the winter is typically expected to slow down these processes (Estrada-Peña and Fernández-Ruiz, Reference Estrada-Peña and Fernández-Ruiz2020). Ticks are only known to search for hosts at temperatures over 7°C (Süss, Reference Süss2008; Namgyal et al., Reference Namgyal, Lysyk, Couloigner, Checkley, Gurung, Tenzin, Dorjee and Cork2021a). However, winter in the Dhar district of Madhya Pradesh is typically dry and cold, with a mean temperature of 15–20°C. In this part of Madhya Pradesh, ticks are more prevalent in rainy seasons due to high temperatures and humidity conditions.
The present study revealed a lack of knowledge among livestock owners on TBDs. The similarities in education and livestock-rearing techniques suggest that these findings may be applicable to other areas and countries (Chakraborty et al., Reference Chakraborty, Steckler, Gronemeyer, Mateus-Pinilla and Smith2023). Ticks and TBDs lead to extensive veterinary and public health issues, particularly in India. TBDs and severe tick infestations have been linked to reduced milk, meat and other animal product output in several developing nations, along with increased animal sickness and death. Ticks transmit a greater number of illnesses than any other blood-feeding arthropod globally, posing a threat to people, their pets and cattle (Rehman et al., Reference Rehman, Conraths, Sauter-Louis, Krücken and Nijhof2019; Ngnindji-Youdje et al., Reference Ngnindji-Youdje, Diarra, Lontsi-Demano, Tchuinkam and Parola2022). Indigenous cow breeds are often believed to have high resistance to ticks and may be reared without proper attention on tick management (Minjauw and McLeod, Reference Minjauw and McLeod2003; Phanchung et al., Reference Phanchung, Dorji, Sonam and Pelden2007; Jonsson et al., Reference Jonsson, Piper and Constantinoiu2014).
Face-to-face interviews in the present study helped to understand farmers' knowledge, their attitudes towards TBDs and acaricide application patterns in fields. The current data indicate majority of farmers to be illiterate and lacking awareness of the TBDs. Most of the farmers used traditional uncemented sheds to maintain their livestock. Besides, the majority of animal owners were using chemical acaricides on their animals without adhering to suitable tick management methods and dosage regime. Animals were classified as having low, moderate or high levels of tick infestation based on the presence of 25, 100 and 150 ticks, as documented by Chigure et al. (Reference Chigure, Sharma, Kumar, Fular, Sagar, Nagar, Upadhaya, Saravanan, Kumar and Ghosh2018). We observed that several farms were severely infected with ticks, leading to a decrease in total productivity. A high negative association was seen between the frequency of acaricide usage and the proportion of tick-infested animals. This suggests that the frequent and effective use of acaricides is a significant factor contributing to the variation in tick prevalence across various farms. Indian researchers determined DLM as the most commonly used acaricide in the field, followed by cypermethrin, amitraz and ivermectin and observed that farms experienced high tick infestation, possibly due to owners' lack of awareness about the correct use of acaricides and the resistance of ticks to the products being used (Ghosh et al., Reference Ghosh, Tiwari, Kumar, Srivastava, Sharma, Kumar, Bandyopadhyay, Julliet, Kumar and Rawat2015; Chigure et al., Reference Chigure, Sharma, Kumar, Fular, Sagar, Nagar, Upadhaya, Saravanan, Kumar and Ghosh2018; Shakya et al., Reference Shakya, Kumar, Fular, Upadhaya, Sharma, Bisht, Nandi and Ghosh2020; Upadhaya et al., Reference Upadhaya, Kumar, Kumar, Sharma, Fular, Bisht, Srivastava, Boruah, Nagar, Shakya and Nath2020). According to Hussain et al. (Reference Hussain, Hussain, Ho, Li, George, Rehman, Zeb and Sparagano2021), out of the livestock owners in the survey, 51 (45.5%) used acaricides frequently, but 49 (43.8%) did not have appropriate disposal methods for spent acaricidal bottles and unused goods, opting to dispose of them in general waste streams, including farm drainage systems. Thirty-four livestock owners, accounting for 30.4% of the total, did not use any acaricides in the year before to our visit, although they had used them previously. Regarding application techniques, 26 farmers (23.2%) used systemic acaricide, while 34 farmers (30.4%) employed topical treatments for tick control. Tesfaye and Abate (Reference Tesfaye and Abate2023) noted that the respondents estimated the amount of the acaricide instead of monitoring doses (whether sprayed or injected) before treatment. In our findings, researchers conducted an investigation that revealed that native breeds were allowed to graze outside, but cross-bred animals were kept confined in a shed. Native breeds have a lower tick infection rate compared to cross-breeds. In many countries that are developing, herd owners acquire acaricide use information from persons without expertise, leading to improper acaricide practices. In the present surveyed places, rural veterinary stores and shop workers with little technical knowledge serve as the primary source of information for farmers, leading to inadequate and improper acaricidal practices. In a prior research conducted in Kenya (Mugambi et al., Reference Mugambi, Wesonga and Ndungu2012), it was shown that many herd owners get information on acaricide administration from untrained vet shop attendants. This lack of sufficient training in animal health care might result in herd owners engaging in harmful practices. Recommendations to farmers were given to rotate the use of acaricides in cattle to reduce acaricide resistance and for cost-effective treatment due to the high frequency of TTBDs (Ghosh and Azhahianambi, Reference Ghosh and Azhahianambi2007).
Most respondents and farmers lack awareness of TBDs and expressed unfavourable attitudes about tick management during face-to-face interviews. Similarly, researchers worldwide shared their views on the knowledge and attitudes of respondents. For instance, Lontsi-Demano et al. (Reference Lontsi-Demano, Laroche, Ngnindji, Djikolmbairangar, Mamoudou and Tchuinkam2021) conducted a cross-sectional survey to evaluate farmers' knowledge and practices regarding ticks and the management of TBDs. They found that herd managers possessed a fundamental understanding of ticks and their impact on animals. Namgyal et al. (Reference Namgyal, Tenzin, Checkley, Lysyk, Rinchen, Gurung, Dorjee, Couloigner and Cork2021b) observed that 128 out of 246 respondents (52%) had sufficient information regarding ticks as carriers of illnesses in people and animals. Hussain et al. (Reference Hussain, Hussain, Ho, Li, George, Rehman, Zeb and Sparagano2021) studied how cattle producers perceive and handle tick infestation. In another study, Hussain et al. (Reference Hussain, Hussain, Ho, Li, George, Rehman, Zeb and Sparagano2021) determined that 47.3% of cattle owners were knowledgeable about TBDs and used sandy flooring, indicating awareness of the related risk factors. In the present study, the most popular animal feeding system is mixed type (57.5%) followed by stall feeding (37.5%). Similarly, Hussain et al. (Reference Hussain, Hussain, Ho, Li, George, Rehman, Zeb and Sparagano2021) noted that 25% of farmers used stall feeding, and 53.6% embraced both methods. Tesfaye and Abate (Reference Tesfaye and Abate2023) reported that the prevalence of tick infestation sometimes increased. Insufficient grazing habitat has caused animal herds to cluster in some areas, resulting in a higher spread of tick infestation.
The present study used the LPT, initially developed by Stone and Haydock (Reference Stone and Haydock1962), and the LIT, developed by Shaw (Reference Shaw1966), to identify and monitor resistance to acaricides. In this study, we found that, all 5 isolates of R. microplus and H. anatolicum collected from 5 different sub-divisions of Dhar district were found to be resistant to DLM which may be due to extensive use of synthetic pyrethroid compounds and easy availability of this compound. Accordingly, the DLM resistance in both the tick species has been reported across the country. For example, Jyothimol et al. (Reference Jyothimol, Ravindran, Juliet, Ajithkumar, Suresh, Vimalkumar, Lenka, Varghese and Ghosh2014) reported comparatively low level of resistance in field tick larvae collected from 2 districts of Kerala. Shyma et al. (Reference Shyma, Gupta and Singh2015) and Gaur et al. (Reference Gaur, Sangwan, Sangwan and Kumar2016) also reported DLM resistance in field ticks collected from Haryana, Rajasthan and Gujarat states of India. Similarly, Kumar et al. (Reference Kumar, Rayulu, Rao and Kumar2017) reported tick larvae from 6 districts of Andhra Pradesh state and reported RF of 1.05–8.78. The ineffectiveness of DLM was also reported from the states like Uttar Pradesh, Assam and Maharashtra at resistance levels I–IV (Chigure et al., Reference Chigure, Sharma, Kumar, Fular, Sagar, Nagar, Upadhaya, Saravanan, Kumar and Ghosh2018; Upadhaya et al., Reference Upadhaya, Kumar, Kumar, Sharma, Fular, Bisht, Srivastava, Boruah, Nagar, Shakya and Nath2020; Khating et al., Reference Khating, Jadhav, Vijay, Sharma, Srivastava, Jadhao, Kumar, Kalwaghe, Siddiqui, Narawade, Dhabale and Chigure2024). DLM resistance has also been reported in R. microplus from West Africa (Adehan et al., Reference Adehan, Biguezoton, Adakal, Assogba, Gbaguidi, Tonouhewa, Kand, Achi, Kagone, Adehan and Mensah2016; Yessinou et al., Reference Yessinou, Akpo, Sidick, Adoligbe, Karim, Akogbeto and Farougou2018), Mexico (Rosario-Cruz et al., Reference Rosario-Cruz, Almazan, Miller, Dominguez-Garcia, Hernandez-Ortiz and de la Fuente2009) and Australia (Gurrero et al., Reference Guerrero, Miller and de León2012). Besides R. microplus, Becker et al. (Reference Becker, Webster, Doyle, Martins, Reck and Klafke2019) reported resistance in R. sanguineus isolate collected from 8 Porto Alegre metropolitan areas, Brazil with RF 1.18–5.67.
Earlier, country-specific discriminating concentration (DC = 2 × LC99) of FIP was determined as 9.6 ppm using LPT against reference susceptible IVRI-I strain of R. microplus (Kumar et al., Reference Kumar, Sharma, Nagar, Rawat, Tiwari, Kumar, Dhakad, Sharma, Saxana, Mehraniya and Singh2016) for differentiating between susceptible and resistant ticks. In the present study, all the collected isolates of R. microplus and H. anatolicum were found susceptible to FIP. This may be due to the high cost and comparatively less use of FIP for tick control. Recently, Shakya et al. (Reference Shakya, Kumar, Fular, Upadhaya, Sharma, Bisht, Nandi and Ghosh2020) characterized 25 isolates collected from 6 states (Madhya Pradesh, Uttarakhand, Meghalaya, Assam, Gujarat and Haryana) and reported RF in the range of 0.39–10.9. Analysing the data, it is observed that FIP is not widely adopted in most of the countries for the management of ticks, and therefore, reports on the development of FIP resistance in tick population are not frequently available in the literature.
Conclusion
The results provide useful insights to aid in the development of educational and outreach programmes that may go beyond the research region. The proper knowledge of TBDs among the animal owners is essential for effective management of tick infestation and improvement of animal health and productivity. The present study to mitigate acaricide resistance and TBDs revealed significant gaps in awareness and proper management strategies. Some farmers showed a basic understanding of tick control, the majority lacked comprehensive knowledge of acaricide resistance and effective disease prevention. Future research should focus on developing targeted educational programmes to enhance farmers' knowledge and attitudes towards sustainable tick control practices. Further, studies should explore other alternatives to chemical acaricides, to minimize acaricide resistance and TBDs in livestock.
Data availability statement
Supplementary data may be provided on request to corresponding author.
Acknowledgements
The authors are grateful to the Hon'ble Vice-Chancellor, Director Research of Nanaji Deshmukh Veterinary Science University, Jabalpur, Dean and Department of Parasitology, College of Veterinary Science & Animal Husbandry (NDVSU), Mhow, Indore (Madhya Pradesh), for providing essential facilities required for the successful completion of the research work.
Author contributions
S. J.: conceptualization, methodology. M. S.: formal analysis, investigation, resources, acquisition, original draft preparation. A. K. J.: conceptualization, supervision. V. A., M. S., A. K. S.: writing – review and editing. G. N. B.: data analysis. G. P. J. and N. J.: writing, review and editing. All authors have read and agreed to the published version of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical standards
The present study was approved by the Institutional Ethical Committee of the College of Veterinary Science & Animal Husbandry (NDVSU), Mhow, Madhya Pradesh, India. All the activities involving individuals were performed following ethical regulations to protect the rights and well-being of the participants (Lodhi et al., Reference Lodhi, Muhammad, Iqbal, Nazir, Ali, Adil, Shamim and Sajid2016) and the data were gathered with consent of the participants.