Introduction
The emergence of metronidazole (MTZ)-resistant isolates of Trichomonas vaginalis (Schwebke et al., Reference Schwebke and Barrientes2006), a causative agent of neglected parasitic infections, is considered a real public health problem (Secor et al., Reference Secor, Meites, Starr and Workowski2014). Trichomonas vaginalis causes a non-viral, sexually transmitted infection (STI) with an incidence of 276 million new cases each year (WHO, 2012). Several complications are associated with trichomoniasis, such as the acquisition and transmission of HIV (Van Der Pol et al., Reference Van Der Pol, Kwok, Pierre-Louis, Rinaldi, Salata, Chen, Van de Wijgert, Mmiro, Mugerwa, Chipato and Morrison2008), pregnancy outcomes, and its relation to prostate and cervical cancers (Menezes et al., Reference Menezes, Frasson and Tasca2016). 5-Nitroimidazole drugs, such as MTZ and tinidazole (TNZ), are the only FDA-approved drugs for the treatment of trichomoniasis. MTZ action occurs through enzymatic pathways, and mechanisms of resistance have already been described (Vieira et al., Reference Vieira, Tasca and Secor2017b). The elevated prevalence of infection has been linked to scarce STI control programmes, and the annual cost to the public health bill in the USA alone to treat trichomoniasis and its associated complications has been estimated to be US$24–167 million (Owusu-Edusei et al., Reference Owusu-Edusei, Chesson, Gift, Tao, Mahajan, Ocfemia and Kent2013).
Phenanthrenes are the secondary metabolites produced by plants from Orchidaceae. Isolated compounds have been investigated for their anticancer, antimicrobial, anti-inflammatory and antioxidant activities (Tóth et al., Reference Tóth, Hohmann and Vasas2017). 1,10-Phenanthroline-5,6-dione (phendione), a phenanthrene-based compound, and its associated metal complexes (metal = Cu2+, Ag+) have been shown to exhibit broad antimicrobial properties (McCann et al., Reference McCann, Coyle, McKay, McCormack, Kavanagh, Devereux, McKee, Kinsella, O'Connor and Clynes2004). In vivo toxicity studies in Galleria mellonella larvae and in mice (acute and chronic toxicity testing) demonstrated that phendione, [Cu(phendione)3](ClO4)2·4H2O (Cu-phendione) and [Ag(phendione)2]ClO4 (Ag-phendione) were well tolerated (McCann et al., Reference McCann, Santos, Silva, Romanos, Pyrrho, Devereux, Kavanagh, Fichtner and Kellett2012). Taking into account the urgency to source new compounds capable of killing trichomonads, the aim of this study was to test the possible anti-T. vaginalis activity of phendione, Cu-phendione and Ag-phendione, as well as to evaluate their selectivity and possible synergism when co-administered with MTZ.
Materials and methods
Compounds
phendione, [Ag(phendione)2]ClO4 (Ag-phendione) and [Cu(phendione)3](ClO4)2·4H2O (Cu-phendione) were prepared in accordance with the methods described in the literature (McCann et al., Reference McCann, Coyle, McKay, McCormack, Kavanagh, Devereux, McKee, Kinsella, O'Connor and Clynes2004) (chemical structures are given in Supplementary Fig. S1).
Culture of T. vaginalis
The assays used T. vaginalis ATCC 30236 and three fresh clinical isolates (TV-LACH4, TV-LACM15 and TV-LACM22) obtained from Laboratório de Análises Clínicas e Toxicológicas, Faculdade de Farmácia UFRGS, Brazil (UFRGS Research Ethical Committee approved the assays under authorization number 18923). In order to verify if the presence of symbiosis with Mycoplasma hominis (MH) and Trichomonasvirus species (TVV) could be related to the test compounds’ susceptibility, isolates were selected according to the following: harbouring TVV only (TV-LACH4), MH only (TV-LACM22), both organisms (ATCC 30236), or not harbouring MH neither TVV (TV-LACM15). Trophozoites were maintained in TYM medium supplemented with heat-inactivated adult bovine serum (10%, v/v) at 37 °C (Diamond et al., Reference Diamond1957). Trichomonads in the logarithmic growth phase with normal morphology were used in the assays.
Minimum inhibitory concentration (MIC) and IC50 determination
Test compounds, MTZ, and the simple metal salts, AgNO3 and CuSO4·5H2O, at decreasing concentrations from 100 µ m (mg L−1 concentration in Table 1), and 2 × 105 trophozoites mL−1 were incubated at 37 °C, 5% CO2 atmosphere for 24 h. After incubation, viability was determined by counting trophozoites with a haemocytometer using trypan blue exclusion dye (0.2%). MIC was determined by inoculation of trophozoites in fresh medium without compounds and analysed for 5 days to confirm the absence of parasite growth (Vieira et al., Reference Vieira, Silva, Menezes, Silva, Silva, Lopes, Macedo, Bastida and Tasca2017a).
Table 1. Anti-Trichomonas vaginalis activity and cytotoxicity effect of MTZ, phendione and its metal complexes
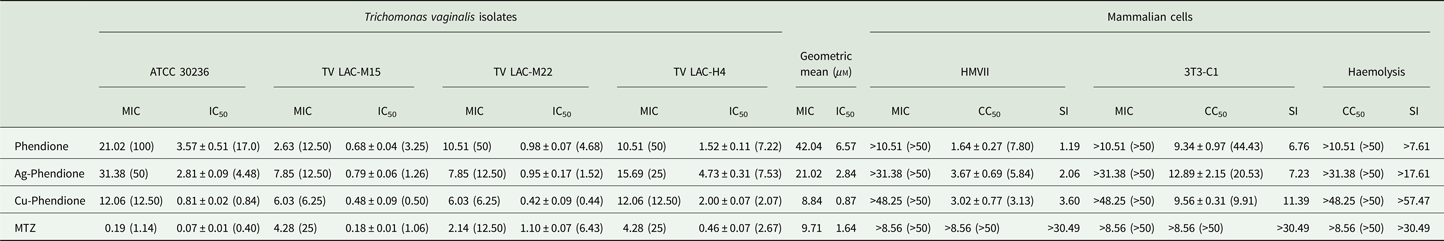
Data are the mean ± s.d. of at least three different experiments performed in triplicate.
All results are expressed in mg L−1 or in μ m (parenthesis), except for SI values.
Effect of compounds on T. vaginalis growth kinetic
Trichomonads (ATCC 30236) at a density of 2 × 105 trophozoites mL−1 were incubated in TYM supplemented with the compounds at MIC and IC50 values. Trophozoites were counted with a haemocytometer at different time periods (from 2 to 120 h).
Cytotoxicity and CC50 determination
HMVII (a tumour lineage from human vaginal epithelial cells) and 3T3-C1 (a non-tumour murine fibroblast lineage) were used. The assay dilution was performed as described above using RPMI and DMEM media, respectively, supplemented with FBS (20%, v/v). After 24 h of exposure, viability was assessed through [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium]bromide (MTT) assay and the formazan produced by viable cell was spectrophotometrically measured at 570 nm (Hübner et al., Reference Hübner, Vieira, Frasson, Menezes, Senger, Santos da Silva, Gnoatto and Tasca2016). MIC was determined comparatively with a negative control (Triton X-100). The selectivity index (SI) for each mammalian cell was calculated based on the ratio CC50/IC50, using the IC50 value calculated by geometric mean among different T. vaginalis isolates.
Haemolytic assay
To evaluate the toxic effect of the test compounds and MTZ on human erythrocytes, haemolysis experiments were performed. The UFRGS Research Ethical Committee approved the assays under authorization CAAE 69979817.5.0000.5347. Erythrocyte suspension (5 × 107 cells mL−1) was incubated with decreasing concentrations of compounds, starting at 50 µ m (mg L−1 concentration in Table 1), for 24 h at 37 °C (Kiss et al., Reference Kiss, Fenyvesi, Bácskay, Váradi, Fenyvesi, Iványi, Szente, Tósaki and Vecsernyés2010). Haemoglobin released into the supernatants was quantified spectrophotometrically at 540 nm. Percentage of haemolysis was compared with 100% for the positive control (Triton X-100, 0.2%). SI was also calculated as described earlier.
Chequerboard assay
Trichomonas vaginalis TV-LACM15 isolate was used to check MTZ and Cu-phendione interaction at concentrations: ¼ × IC50, ½ × IC50, IC50, 2 × IC50 and 4 × IC50. Fractional inhibitory concentration index (FICI) was estimated using the following formula: FICA + FICB = FICI, where FICA is the value of Cu-phendione in the combination/value of Cu-phendione alone and FICB is the value of MTZ in the combination/value of MTZ alone. The interaction was classified as ‘synergy’ if FICI ⩽ 0.5, ‘antagonism’ if FICI > 4.0 and ‘no interaction’ if FICI = 0.5–4.0 (Odds et al., Reference Odds2003).
Statistics
All experiments were performed in triplicate with three independent cultures (n = 3). Statistical analysis used Student's t-test with the 5% level of significance being applied to data. IC50 and CC50 values were calculated using the GraphPad Prism6 software (San Diego, CA) by nonlinear regression.
Results
Anti-T. vaginalis activity
Table 1 summarizes the MIC and IC50 values obtained after the treatment (24 h) of different T. vaginalis isolates, which present distinct phenotypic backgrounds, with MTZ, phendione, Ag-phendione and Cu-phendione. Overall, all of the test compounds had a strong effect on trophozoite viability, displaying low MIC and IC50 values, and with Cu-phendione having the highest anti-T. vaginalis activity. Corroborating these results, the anti-T. vaginalis activity of the compounds (at their MIC and IC50 values) was evidenced from kinetic curves (Fig. 1), and 4 h was sufficient to observe significant differences in proliferation rates between untreated and treated trophozoites. The simple metal salts, AgNO3 and CuSO4·5H2O, were ineffective against the trophozoites (data not shown).
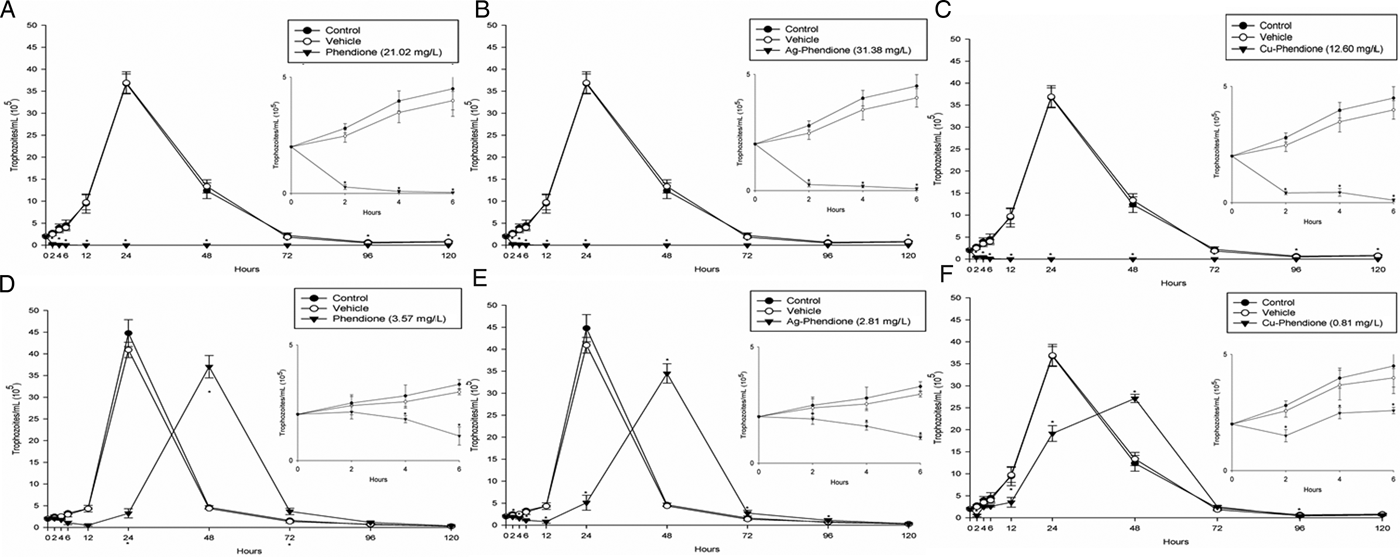
Fig. 1. Growth kinetic curves of ATCC 30236 T. vaginalis isolate in the presence of: phendione MIC (A) and IC50 (D); Ag-phendione MIC (B) and IC50 (E); and Cu-phendione MIC (C) and IC50 (F). Treated trophozoites were compared with control (untreated). Insets show data at 0 to 6 hours in detail. Data are the mean ± s.d. of at least three different experiments (parasite suspensions) performed in triplicate. *Means statistical difference from controls (P < 0.05).
Cytotoxicity assays
Cytotoxicity against HMVII and 3T3-C1 cell lines is shown in Table 1. Cu-phendione showed the highest SI for both erythrocytes (>57.47) and the non-tumour cell lineage (11.39), demonstrating both selectivity towards the parasite and the safety of the complex.
Synergy potential of Cu-phendione with MTZ
Considering the higher MTZ resistance presented by TV-LACM15 (MIC = 4.28 mg L−1) when compared with the ATCC 30236 isolate (MIC = 0.18 mg L−1), TV-LACM15 was used to test the Cu-phendione and MTZ association. The chequerboard assay revealed a synergistic effect (FICI ⩽ 0.5) upon co-administration of Cu-phendione and MTZ (Table 2).
Table 2. FICI data for T. vaginalis TV-LACM15 isolate for each combination tested
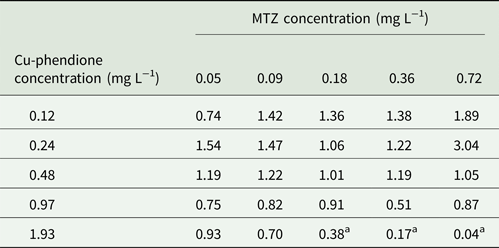
a Synergy interaction by Odds (Reference Odds2003).
Discussion
The treatment of trichomoniasis relies on a single class of drugs, 5-nitroimidazoles, which present several adverse effects and are failing due to emerging resistance. Studies demonstrated an inefficient effect of MTZ, result of adverse reactions that decrease or impair treatment adhesion by the patient. Cases of hypersensitivity reaction were already described leading to severe anaphylactic reactions and disulfiram-like alcohol intolerance, beyond the commonly effect of headache, nausea, vertigo, vomiting, diarrhoea and a metallic taste (Kissinger, Reference Kissinger2015). Moreover, progress of resistance to the 5-nitroimidazole class is spread worldwide and is of concern for the public health. A recent study in the USA demonstrated that association between trichomoniasis and HIV infection is a burden to the health system, with costs reaching $167 million per year (Chesson, Reference Chesson, Blandford and Pinkerton2004).
Scientific development in new alternatives for the treatment of trichomoniasis is based on natural and synthetic compounds in order to discover new promising activity. Vieira et al. (Reference Vieira, Giordani, Macedo and Tasca2015) gathered different sources of molecules currently studied, including those derived from marine products and other from native florae, such as plants of the Brazilian Caatinga bioma and used by indigenous tribes. These natural compounds or semisynthetic derivatives generate about 35% of the new approved drugs (Newman, Reference Newman and Cragg2012). Concerning about synthetic products against T. vaginalis, Bala & Chhonker, (Reference Bala and Chhonker2018) brought together several trichomonicidal derivatives of 5-nitroimidazoles, benzimidazole, amine, isatin, in addition to agents already approved and microbicide with spermicidal or antifungal properties.
Metal-based drugs are already used in therapies as cisplatin with platinum for cancers’ treatment and gold-based drugs as auranofin in rheumatoid arthritis (Allardyce & Dyson, Reference Allardyce and Dyson2016). Indeed, auranofin has been demonstrated anti-T. vaginalis effectiveness in vitro and in vivo, presenting IC50 values of 0.4–2.5 µ m (Hopper et al., Reference Hopper, Yun, Zhou, Le, Kehoe, Le, Hill, Jongeward, Debnath, Zhang, Miyamoto, Eckmann, Kirkwood and Wrischnik2016). In this context, we highlight the metal-based compound Cu-phendione, which demonstrated high activity against this parasite, with MIC value of 8.84 µ m and IC50 0.87 µ m, and more effective than MTZ.
Phendione and its metal complexes have previously demonstrated antimicrobial properties against Candida albicans yeast (Eshwika et al., Reference Eshwika, Coyle, Devereux, McCann and Kavanagh2004), the multi-resistant mould Scedosporium apiospermum (McCann et al., Reference McCann, Santos, Silva, Romanos, Pyrrho, Devereux, Kavanagh, Fichtner and Kellett2012), dematiaceous Phialophora verrucosa (Granato et al., Reference Granato, Gonçalves, Seabra, McCann, Devereux, Dos Santos and Kneipp2017) and the Gram-negative bacterium Pseudomonas aeruginosa (Viganor et al., Reference Viganor, Galdino, Nunes, Santos, Branquinha, Devereux, Kellett, McCann and Santos2016). Herein, we have demonstrated the potent activity of this class of compound against T. vaginalis. Table 1 shows IC50 values very close to or lower than that of the clinical drug, MTZ. Each T. vaginalis isolate used has characteristics previously evaluated by Becker et al. (Reference Becker, dos Santos, Frasson, Rigo, Macedo and Tasca2015), such as the occurrence of symbiosis infections with MH and TVV. As expected, no relation between anti-T. vaginalis activity and symbiosis was found, confirming the antiparasitic activity against several isolate types. Growth kinetics demonstrated that an exposure time of only 4 h was necessary to reduce the number of viable trophozoites, and at 12 h there was a complete cessation of parasite proliferation at the MIC values. The greater activity of the metal–phendione complexes, compared with the simple metal salts (AgNO3 and CuSO4·5H2O) and the metal-free phendione ligand, against T. vaginalis trophozoites highlights the necessity for elucidating their mechanisms of action in further studies.
The pathogen T. vaginalis initiates the infection by recognition of host cells, a process mediated by cysteine proteases located on the parasite surface. Once in the site of infection, trophozoites alter their conformation from pyriform to amoeboid for cytoadherence. This tight association supports the process of tissue damage to ensure their survival by acquisition of nutrients, as iron and lipids, from erythrocytes (Menezes et al., Reference Menezes, Frasson and Tasca2016). Thus, investigating the cytotoxicity of bioactive compounds against cells involved in the infection is crucial. The present compounds presented a low SI against HMVII vaginal epithelial cells, as expected, since earlier studies showed them to have exceptional in vitro anti-cancer activity (McCann et al., Reference McCann, Santos, Silva, Romanos, Pyrrho, Devereux, Kavanagh, Fichtner and Kellett2012). In tests using the erythrocytes and the non-tumour cell line 3T3-C1, Cu-phendione was well tolerated and comparable with the reference drug, MTZ, which reveals pharmacological selectivity. Furthermore, phendione and its metal complexes caused no haemolysis. These preliminary results suggest that the compounds, particularly Cu-phendione, were well tolerated in vitro by host cells.
Cu-phendione, the compound with the highest anti-T. vaginalis activity and the best SI, was selected to check for synergistic interactions with MTZ using the chequerboard assay. A synergy effect was observed using Cu-phendione (⩾1.93 mg L−1) in combination with MTZ (⩾0.18 mgL−1), within a range of drug concentrations that are well tolerated by mammalian cells (Table 1). These results demonstrate that the interaction between both compounds reduces the concentration required to cause parasite death and suggest different sites of action for the two compounds. Consequently, distinct mechanisms may be involved in cell death thus enabling evasion of the MTZ resistance pathways.
There is an urgent necessity for new therapeutics capable of effectively treating trichomoniasis and severing the link between T. vaginalis infection and HIV transmission. In this context, Cu-phendione offers credible potential against the proliferation of T. vaginalis. Future studies will focus on the elucidation of the mechanism(s) of action of these compounds.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003118201800152X.
Financial support
This study was supported by grants from the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Marine Biotechnology Program (Rede Mar Ativo, grant 408578/2013-0), Fundação de Apoio à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) PRONEM (grant 16/2551-0000244-4), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ). G.V.R. thanks CNPq for fellowship. T.T. thanks CNPq for research fellowship (grant 312292/2017-1).
Conflicts of interest
None.
Ethical standards
The T. vaginalis fresh clinical isolates were obtained from Laboratório de Análises Clínicas e Toxicológicas, Faculdade de Farmácia UFRGS, Brazil (UFRGS Research Ethical Committee approved the assays under authorization number 18923) and the human erythrocytes for the haemolysis experiments were obtained from healthy volunteers (UFRGS Research Ethical Committee approved the assays under authorization CAAE 69979817.5.0000.5347).






