Introduction to multi-disciplinarity of parasitology
It is well-known that parasitology is a broad and far reaching discipline, constantly changing in its remit and priorities, and typically set within medical, veterinary or wildlife fields of research (Stothard and Rollinson, Reference Stothard and Rollinson2018). Academic interest aside, much of the future ambition and direction of today's practicing parasitologists can be found within the 2030 Sustainable Development Goals (SDGs), especially those working on malaria and neglected tropical diseases (Engels, Reference Engels2016). These goals have a strong ethos of reducing inequities in human health across the globe, alongside more sensible management of the world's finite resources (Fitzpatrick and Engels, Reference Fitzpatrick and Engels2016). Although parasites ‘drain’ ecosystems, their presence is an inevitability, argued perhaps even as an essentiality within a healthy ecosystem (Vannatta and Minchella, Reference Vannatta and Minchella2018). Implicit in the SDGs, however, is the recognition that solving global problems typically requires multi-disciplinary teams (Bangert et al., Reference Bangert, Molyneux, Lindsay, Fitzpatrick and Engels2017). These teams seek to forge essential ‘cross-talk’ among critical components of knowledge to assemble a holistic appraisal of a solution over and above what simple reductive reasoning within each discipline alone could achieve.
Today, fostering a multi-disciplinary approach is fashionable and a growing priority of national and international funding and development agencies (Brown et al., Reference Brown, Deletic and Wong2015; Rylance, Reference Rylance2015). It may be that key individuals, as exemplified by the Victorian explorer and polymath Richard Francis Burton (1821–90), are talented enough, by themselves, to bring diverse disciplines together, coherently, e.g. anthropology, linguistics, cartography and natural history (Pettitt, Reference Pettitt2015); more usually, however, it is the assembly of multi-disciplinary research teams that include specialist individuals who are open to cross-talk and exchange of ideas across wider perspectives. A good example is the implementation research consortium of COUNTDOWN that has brought together parasitologists, social scientists and health economists to tackle issues surrounding the scale-up of preventive chemotherapy in sub-Saharan Africa (Stothard et al., Reference Stothard, Kabatereine, Archer, Al-Shehri, Tchuem-Tchuente, Gyapong and Bustinduy2017). Another is the multi-disciplinary programmes within the Zoonoses and Emerging Livestock Systems (ZELS) initiative, supported by several major UK funding agencies, which aim to promote and develop One Health concepts in which parasitology has a featured role (Blake and Betson, Reference Blake and Betson2017; Kingsley and Taylor, Reference Kingsley and Taylor2017). The word cloud shown in Fig. 1 is a single snap shot of this multi-disciplinary vista.

Fig. 1. A word cloud exploring many of the topics, disciplines and activities that can be included within the study of parasites and parasitological research. The list is not exhaustive and likely has several omissions as the discipline of parasitology moves forward.
It could be argued that much of the principles of multi-disciplinary approaches can be traced back to the wider remit of fundamental parasitology that sought to unravel the dynamic and evolutionary connections between parasites and their hosts, both in time and space, well before the concept of One Health was formalized. Its origin lies within the approach taken by the eminent helminthologist Robert Thomson Leiper (1881–1969) (cf. Cox, Reference Cox2017a, 2017Reference Coxb), although he was not well-known for his team spirit as his publication list as single author might testify (Stothard et al., Reference Stothard, Kabatereine, Archer, Al-Shehri, Tchuem-Tchuente, Gyapong and Bustinduy2017). Nevertheless, modern parasitology has not been given the full recognition that it deserves for embracing the tools, techniques and approaches first developed in other fields, albeit in qualitative or quantitative methods, and then applying them to the study of parasites with subsequent inter-disciplinary cross-talk.
For example, parasitologists have provided insights at the molecular level, by exploring the myriad of immuno-physiological adaptations and implications of parasites’ novel lifestyles and the perturbation of associated host microbial fauna (Jackson et al., Reference Jackson, Friberg, Little and Bradley2009; Leung et al., Reference Leung, Graham and Knowles2018); or they have reconstructed deep parasite histories through analyses of DNA sequences to reveal their intricate phylogenetic pathways and connection with geological vicariances and/or ecological landscapes (Johnson et al., Reference Johnson, Adams, Page and Clayton2003; Cable et al., Reference Cable, Barber, Boag, Ellison, Morgan, Murray, Pascoe, Sait, Wilson and Booth2017). In an even broader perspective and sometimes speculative or predicative in manner, understanding the populational behaviours of potential or actual parasites and their hosts has been developed. An example is the related studies on the domestication of animals, with later farming and agricultural practices, which created new opportunities for parasites to exploit our altered environments for zoonoses or anthropozoonoses (Mennerat et al., Reference Mennerat, Nilsen, Ebert and Skorping2010). In addition, these changes also need to be framed within the context of global climate change (Booth, Reference Booth2018).
The ancient origins of parasites
It could be said that all parasites have a fascinating evolutionary history which may also provide some key clues about the histories of the evolution of their host(s). Understanding parasite associations, diversity and distributions through deep time relies almost exclusively on the methods of inference and, more rarely, direct evidence from fossils. Cophylogenetic studies, comparing and mapping host and parasite evolutionary histories, can track associations between codiverging organismal lineages, revealing host dependencies, host switches and the origins of parasitic interactions and major evolutionary transitions (Page, Reference Page1994). Mapping (usually present day) ecological, morphological and developmental traits and geographical distributions provides a rich ground for inferring finer details of host–parasite associations and change through time (Krasnov et al., Reference Krasnov, Shenbrot, Khokhlova and Degen2016). Because of the indirect nature of these historical inferences they remain hypotheses. However, where direct evidence of host–parasite associations and interactions can be revealed from fossils, the opportunity to test hypotheses, calibrate phylogenies and reveal entirely novel host–parasite interactions arises. Such opportunities are becoming increasingly tractable and attractive with sophisticated tools such as diagnostic imaging and genomic analyses (De Baets and Littlewood, Reference De Baets and Littlewood2015b).
Enhanced imaging techniques, coupled to computed-tomographic reconstructions, are revealing unusual and hitherto unseen details within the animal fossil record at an increasing rate. The presence and nature of parasitic associations are also coming into relief through these methods. From small mites to larval pentastomids and isopod crustaceans, exquisite fossils of arthropod parasites have been revealed from fossilized vertebrate and invertebrate hosts (De Baets and Littlewood, Reference De Baets and Littlewood2015a); fossils of metazoan parasites date all the way back to the Cambrian (Bassett et al., Reference Bassett, Popov and Holmer2004). Amber-entombed parasites and vectors have long been a source of detailing historical records of intimate parasite interactions in terrestrial systems, but such findings are rare and are restricted to very few geological deposits (for nematodes, see Poinar, Reference Poinar2015).
More recent (<10 000 year-old) fossil evidence of parasites is becoming increasingly tractable for DNA research and is a focus of growing archaeological interest. Importantly, although not-lithified and, strictly speaking, occurring as ‘sub-fossils’, archaeological discoveries such as mummies, and human settlements, particularly including burial grounds or latrines, are yielding morphologically identifiable helminth egg deposits (Bouchet et al., Reference Bouchet, Guidon, Dittmar, Harter, Ferreira, Chaves, Reinhard and Araújo2003), some of which have been shown to be amenable to DNA sequencing (Côté et al., Reference Côté, Daligault, Pruvost, Bennett, Gorgé, Guimaraes, Capellii, Le Bailly, Geigl and Grange2016). Identification of helminth eggs point to diet, likely infection intensities and disease, associations with domestic animals and possible trade or migration patterns of those parasitized.
A modern focus on genomics via next generation sequencing of common human helminths has assisted with ancient (a)DNA work on such deposits; complete genomes from modern isolates provide a bioinformatic reference scaffold from which to retrieve and characterize ancient human and livestock parasite sequences (Søe et al., Reference Søe, Nejsum, Seersholm, Fredensborg, Habraken, Haase, Hald, Simonsen, Højlund, Blanke, Merkyte, Willerslev and Kapel2018). The opportunity to reveal genetic and genomic sequences in the distant past can provide direct evidence of historical baseline data, ancient genetic haplotypes and pre-intervention genetic signatures for both parasites and vectors. Little archaeological material has suitably preserved aDNA for sequencing, but the opportunity to retrace historical genetic variants directly through space and time adds context to present-day epidemiological data and links modern genomic data both analytically and evolutionarily. Ideally, tracking parasites through time provides insights into parasites origins, patterns of diversification and major transitions, until a species dies out.
Indeed, palaeontology and even planetology teaches us that species extinction is inevitable. The fossil record provides ample evidence of the existence of species no longer alive today; the process of extinction is both alive and well. Records of biodiversity over the last 200 years and present-day surveys attest to the fact that species continue to die out – increasingly so at an alarming rate as we see the impacts of the Anthropocene defining a very different natural world. Parasites are no different in this arena, although human intervention targets agents of human disease in control, elimination and eradication programmes. Whether through active intervention, as, for example, in the concerted efforts to eradicate the Guinea worm (Dracunculus medinensis) (Hopkins et al., Reference Hopkins, Ruiz-Tiben, Eberhard, Roy and Weiss2017), or more usually through the associated extinction of their hosts, or through ecological perturbations, parasites come and go (Carlson et al., Reference Carlson, Burgio, Dougherty, Phillips, Bueno, Clements, Castaldo, Dallas, Cizauskas, Cumming, Doña, Harris, Jovani, Mironov, Muellerklein, Proctor and Getz2017). There is no doubt that parasites will remain on the Earth long after the extinction of mankind or remain with us upon our future diaspora, either inadvertently within us or upon our contaminated itinerant machines.
Parasitology for today and the future
So, what is the multi-disciplinarity of today's parasitology? Frank Cox, a former editor of this journal, once remarked that it is easier to define what parasitology isn't rather than what it actually is (Cox, Reference Cox2009). Yet, the simple statement that ‘all living species are involved in parasitism, either as parasites or as hosts’ is a universal truth. This sets the founding concept for discussions at the 2017 Autumn Symposium of the British Society for Parasitology entitled ‘The multidisciplinarity of parasitology: host-parasite evolution and control in an ever-changing world’. Without doubt, as a way of life, parasitism is a successful evolutionary strategy, but is also part of a broader picture of symbiosis and a convenient classification of the dynamics of how organisms, big or small, interact. As a metaphor, it is tremendously powerful and is regularly used in today's language to describe significant socio-political events as societies and even nations sometimes negatively exploit others. The agenda of parasitology is exciting, challenging and globally relevant.
Like the agenda for the BSP Autumn Symposium, the grouping of the papers assembled into this special issue revolves around three themes, but these divisions blur, as they should, for we encourage cross-talk as much as possible. The ‘ever changing world’ hopes to place parasitological research within the new terminology of the Anthropocene and how the mankind is altering global environments which may or may not favour parasitic diseases of medical, veterinary or wildlife importance. The ‘multi-disciplinarity of parasitology’ encourages synergies between molecular, ecological and social science components that link parasites and hosts into a more holistic appraisal of parasitism. The meeting closes upon ‘host–parasite evolution and control’ to recognize that parasites are not simple self-replicating automata and are very able to respond rapidly to interventions waged against them. It was very fitting to discuss this aspect of parasitism at the BSP Autumn Symposium in the meeting rooms of the Linnean Society for it was at this Society that the papers of Charles Darwin (1809–1882) and Alfred Russel Wallace (1823–1913) were presented, nearly 160 years ago, on the origins of speciation by natural selection.
An ever-changing world
In line with the 2030 SDGs, there is a drive towards universal health access, such that the well-being of all is promoted as much as possible. Attaining well-being typically infers the absence of preventable disease, none more so than the lasting control of several neglected tropical diseases. In this light, the drive towards interruption of parasite transmission and disease elimination is important, but requires more careful orchestration of available resources within the health system. Molyneux et al. (Reference Molyneux, Dean, Adekeye, Stothard and Theobald2018) provided a thought-provoking review which considers the perspectives of policy makers, managers and frontline health workers and how their actions should be better integrated or harmonized on each of their targeted diseases (Molyneux et al., Reference Molyneux, Dean, Adekeye, Stothard and Theobald2018). A good example of the progress being waged against urogenital schistosomiasis in Cameroon is provided by Tchuem Tchuenté et al. (Reference Tchuem Tchuenté, Eloundou Ombede, Dongmo Noumedem, Djomkam Chuinteu, Fesuh Nono, Nguepkap Lemegne, Femoe Membe, Feussom Gipwe, Kenfack, Ngang, Ndonou Tchoumdop, Cunningham and Stothard2018). The well-known Crater Lake villages of Barombi Mbo and Barombi Kotto offer context-specific exemplars in the decline of Schistosoma haematobium infection. Moreover, interventions undertaken at these locations, such as exploration of better community engagement or how complementary interventions, including health education and snail control, can work alongside preventive chemotherapy approaches for more sustained reductions in schistosome transmission (Tchuem Tchuenté et al., Reference Tchuem Tchuenté, Eloundou Ombede, Dongmo Noumedem, Djomkam Chuinteu, Fesuh Nono, Nguepkap Lemegne, Femoe Membe, Feussom Gipwe, Kenfack, Ngang, Ndonou Tchoumdop, Cunningham and Stothard2018).
In parallel to the battles being waged against human parasites, those against parasitic helminths in livestock have some unique challenges, especially when the economic value of farmed ruminants is considered. Vercruysse et al. (Reference Vercruysse, Charlier, Van Dijk, Morgan, Geary, Von Samson-Himmelstjern and Claerebout2018) discuss the future of worm control on farms and point out that four facets need to be considered: (1) development and application of better diagnostic tools, (2) implementation of innovative control approaches based on anthelminthic vaccines and selective breeding of resistant stocks, (3) sustainable use of existing anthelminthic drugs alongside introduction of new compounds and (4) rational integration of future control practices within a holistic information the animal production systems (Vercruysse et al., Reference Vercruysse, Charlier, Van Dijk, Morgan, Geary, Von Samson-Himmelstjern and Claerebout2018). The connection between human and animal health and that of farmed livestock and irrigated agriculture areas is well exemplified by human and animal fascioliasis. Mas-Coma et al. (Reference Mas-Coma, Bargues and Valero2018) highlighted recent research on the liver fluke, discussing the subtleties of parasite epidemiology, including the initial infection risk of how parasite metacercariae can be ingested on a variety of food items as well as in drinking water. The former is becoming increasingly important as a burgeoning variety of freshwater wild plants are being consumed by people as often being sold in uncontrolled urban markets. The changing patterns of Fasciola infection is another factor adding to the increasing diversity of transmission routes of human fascioliasis across the world (Mas-Coma et al., Reference Mas-Coma, Bargues and Valero2018).
Multi-disciplinarity of parasitology
With the introduction of molecular DNA methods, the detection and diagnosis of several parasitic diseases has moved away from classic parasitological sampling, such as the detection of parasite ova under a microscope to more formal use of parasite-specific genetic markers. With advances in next generation DNA sequencing, Le Clec'h et al. (Reference Le Clec'h, Chevalier, McDew-White, Allan, Webster, Gouvras, Kinunghi, Tchuem Tchuenté, Garba, Mohammed, Ame, Webster, Rollinson, Emery and Anderson2018) explored the use of whole genome amplification and exome sequencing of archived schistosome miracidia. Inspection of sequences retrieved by this method facilitates the transition from population genetics, using limited numbers of markers, to population genomics that offer a much wider insight into genome-wide marker associations. Analysis of these data can offer new insights into the pathogenicity of novel genetic variants and also augment the importance of museum-based collections of parasite material, such as the Schistosome Collection At the Natural History Museum (SCAN), as a collective resource for sharing across divergent research groups in future (Le Clec'h et al., Reference Le Clec'h, Chevalier, McDew-White, Allan, Webster, Gouvras, Kinunghi, Tchuem Tchuenté, Garba, Mohammed, Ame, Webster, Rollinson, Emery and Anderson2018).
The question remains, however, which detection method is optimal, and can the best method deliver meaningful results to control programmes that have to make rapid public health decisions. Al-Shehri et al. (Reference Al-Shehri, Koukounari, Stanton, Adriko, Arinaitwe, Atuhaire, Kabatereine and Stothard2018) highlighted that there is no single diagnostic method to be used for the detection of S. mansoni infection, but rather a more careful consideration of and application of multiple suitable methods. In particular settings, it is logical to continue with traditional parasitological methods, while in others DNA-based method should be used (Al-Shehri et al., Reference Al-Shehri, Koukounari, Stanton, Adriko, Arinaitwe, Atuhaire, Kabatereine and Stothard2018). The choice and cost of which DNA assay to be used for the detection of schistosomiasis and soil-transmitted helminthiasis is also discussed by Cunningham et al. (Reference Cunningham, Stothard, Osei-Atweneboana, Armoo, Verweij and Adams2018). A novel low-cost screening method, making use of multiplex high-resolution melt curve analysis, shows much promise; it can be used as a rapid screening tool to process a large number of faecal specimens, and is of particular use when infection prevalence is expected to be low (<10%) (Cunningham et al., Reference Cunningham, Stothard, Osei-Atweneboana, Armoo, Verweij and Adams2018).
Use of molecular markers, such as DNA barcodes, has application in environmental monitoring of schistosome infections and transmission. Using a combination of genetic loci, the detection of natural hybrids between S. haematobium and S. bovis has been evidenced in the Senegal River Basin (Séne et al., Reference Séne, Rollinson and Webster2018). With more careful sampling of miracidia obtained from infected patients, Séne et al. (Reference Séne, Rollinson and Webster2018) revealed many hitherto unknown, fine scale heterogeneities in transmission of schistosomes between humans and livestock. Further surprises in the transmission of schistosomiasis on Zanzibar are evidenced by Pennance et al. (Reference Pennance, Shaali, Amour, Suleiman, Allan, Rollinson and Webster2018); their careful molecular characterization of schistosome cercariae shed from infected Bulinus on Pemba confirmed autochthonous transmission of S. bovis (Pennance et al., Reference Pennance, Shaali, Amour, Suleiman, Allan, Rollinson and Webster2018).
The repertoire of methods for the detection of Old World cutaneous leishmaniasis is surprisingly small, and the search for novel biomarkers continues. Subramaniam et al. (Reference Subramaniam, Austin, Schocker, Montoya, Mesri, Al-Salem, Almeida, Michael and Acosta-Serrano2018) have identified five candidate neoglycoproteins (NGPs): Galα (NGP3B), Galα(1,3)Galα (NGP17B), Galα(1,3)Galβ (NGP9B), Galα(1,6)[Galα(1,2)]Galβ (NGP11B) and Galα(1,3)Galβ(1,4)Glcβ (NGP1B) which are differentially detected in sera from individuals with Leishmania major infection (Subramaniam et al., Reference Subramaniam, Austin, Schocker, Montoya, Mesri, Al-Salem, Almeida, Michael and Acosta-Serrano2018). The future use of the NGPs adapted into immune-lateral flow strips, for example, holds promise for the identification of infection as well as longer term markers of treatment cure.
Host–parasite evolution and control
Unlike the well-studied genus Schistosoma in Africa, lung flukes of the genus Paragonimus have received very little attention. This is due in part because of the more isolated foci of transmission and difficulties in capturing infected hosts within the parasite's lifecycle that involves both snails, freshwater crustaceans and humans (Cumberlidge et al., Reference Cumberlidge, Rollinson, Vercruysse, Tchuem Tchuenté, Webster and Clark2018); indeed, there are perhaps more questions than answers concerning the transmission of paragonimiasis in West and Central Africa. The importance of riverine prawns in Africa has recently received increased attention as a potential biological method of population control of intermediate host snails of schistosomiasis. Diakité et al. (Reference Diakité, N'Zi, Ouattara, Coulibaly, Saric, Yao, Hattendorf, Utzinger and N'Goran2018) conducted a detailed ecological study to show that negative association between intermediate host snail densities and riverine prawns could occur. However, no pattern between this relationship and the prevalence of human schistosomiasis could be found (Diakité et al., Reference Diakité, N'Zi, Ouattara, Coulibaly, Saric, Yao, Hattendorf, Utzinger and N'Goran2018).
While falciparum-malaria is a major blight on human health across the world, control of all forms of malaria should not be ignored, especially when elimination targets are proposed. Furthermore, there are still components of the lifecycle of Plasmodium that remain to be fully clarified. For example, gametocytes represent the sexual stage of the parasite and are indispensable for the transmission of the parasite from human to mosquito; yet, this is perhaps the least understood stage in the parasite's life cycle, and the current state of knowledge is reviewed (Beri et al., Reference Beri, Balan and Tatu2018). In terms of the life of Plasmodium vivax, it is a curious fact of the evolution of the hypnozoite theory of malarial relapse is its transmogrification from theory into ‘fact’ (Markus, Reference Markus2018). However, Markus (Reference Markus2018) goes on to describe that relapse needs to take into account the possibility of a dual or multiple extra-vascular origin(s) of P. vivax in non-reinfection recurrences. This aspect raises the importance of developing better treatment schedules for patients that have putative infections who may later go on to transmit infections to mosquitoes by cryptic relapse mechanisms, perhaps recrudescing local transmission in mosquitoes.
The final paper closes this special issue upon consideration of the importance of monitoring mosquitoes themselves as a future means of shedding light on transmission patterns of lymphatic filariasis and malaria. With regard to lymphatic filariasis, xenomonitoring is recommended, particularly in the context of elimination of transmission (Opoku et al., Reference Opoku, Minetti, Kartey-Attipoe, Otoo, Otchere, Gomes, de Souza and Reimer2018). However, these authors go on to describe and compare the efficiency of collection techniques using an Anopheles gravid trap (AGT) against other better-known methods e.g. indoor resting collection and pyrethrum spray catches. The AGT method, as applied in Ghana, showed high trapping efficiency in collection of the highest mean number of anophelines per night as well as good trapping potential for collecting Anopheles melas. This latter species is notoriously difficult to catch using existing methods. The AGT method appears appropriate for xenomonitoring of lymphatic filariasis and malaria vectors (Opoku et al., Reference Opoku, Minetti, Kartey-Attipoe, Otoo, Otchere, Gomes, de Souza and Reimer2018).
Celebrating collaboration and a parasitological career
An underlying theme of the BSP Autumn Symposium on parasitism was also to explore mutualism, those biological interactions seen to benefit all players. Mutualism might be considered the antithesis of parasitism, but we should note that our Symposium was each supported in part by The Linnean Society of London, The Royal Society of Tropical Medicine and Hygiene, The London Centre for Neglected Tropical Diseases and The International Federation for Tropical Medicine (IFTM). Without too much coaxing, each society provided much more than goodwill by covering the costs necessary to run the meeting; this was a perfect example of the power of forging collaboration across groups towards the pursuit of a common good or goal.
Broadly speaking, research collaboration(s) often begin upon finding individuals whom are keen to share their wisdom and skills and mentor across the broader network and beyond. With the award of IFTM Medal at the BSP Autumn Symposium, we were able to celebrate the career of Professor David Rollinson, FLS and a former President of the BSP, who has been active in parasitological research for over 40 years, Fig. 2. David also recently received the Linnean Society Gold Medal, in recognition of his services to science. By following his example, our BSP Autumn Symposium and associated Special Issue volume of Parasitology seeks to encourage others to devote their careers and efforts into parasitological research, engendering the spirit of collaboration throughout.
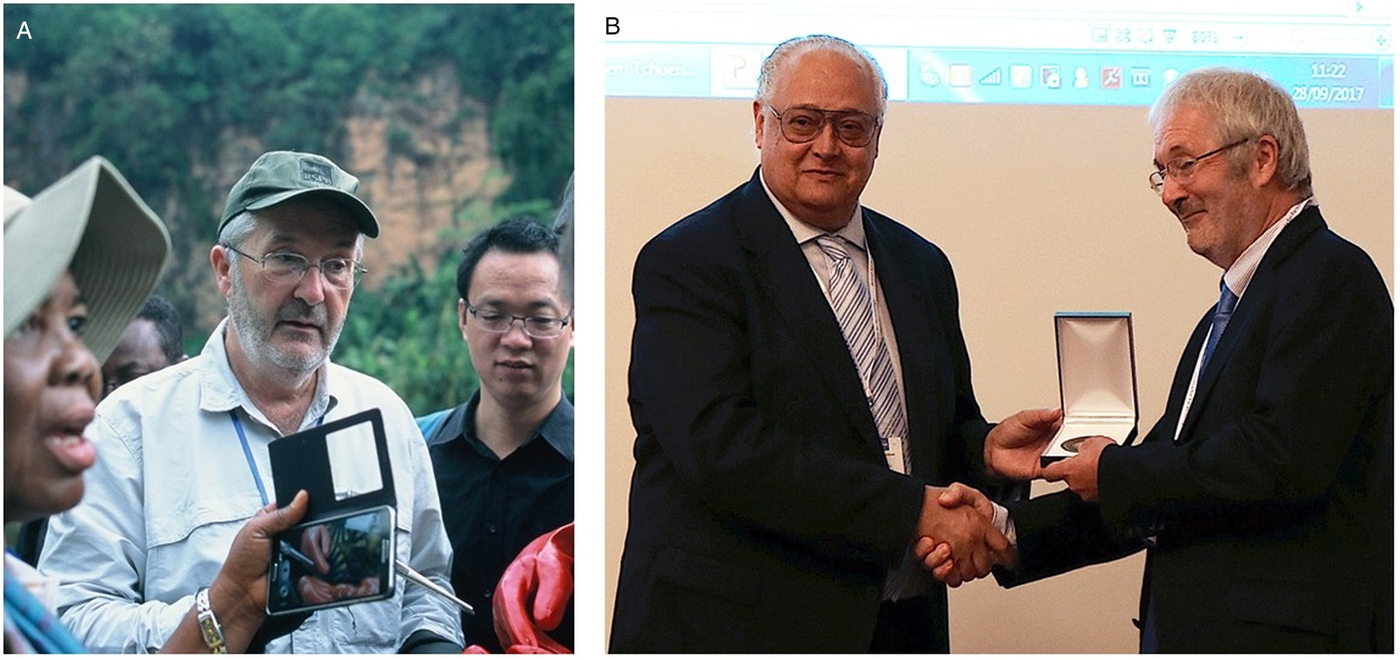
Fig. 2. Celebrating the achievements of David Rollinson, FLS within multi-disciplinary parasitological research. (A) David overseeing training in malacology and parasitology fieldwork in Cameroon. (B) Receiving the IFTM medal at the BSP Autumn Symposium from IFTM President Santiago Mas-Coma in recognition of his longstanding career and advancing international collaborations within parasitology.
Acknowledgements
We thank the British Society for Parasitology (BSP), The Linnean Society and The Royal Society for Tropical Medicine and Hygiene and Cambridge University Press for their contributions to the 2017 BSP Autumn Symposium. We gratefully acknowledge the efforts of our invited speakers, especially those who travelled from afar, our speed poster presenters as well as those participants and attendees who made this meeting convivial, and who have also contributed to this special issue. We warmly thank Professor John Ellis for his editorial assistance and smooth management in preparing this special issue for production. We congratulate Professor David Rollinson on his receipt of the IFTM Medal and look forward for many future years in continuing his research. As Director of COUNTDOWN, JRS gratefully acknowledges funding from Department for International Development (DFID) that encourages multi-disciplinary implementation research.





