Published online by Cambridge University Press: 02 November 2021
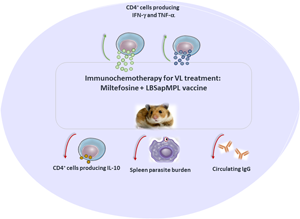
The control of human visceral leishmaniasis (VL) is hard since there are no vaccines available as well as the treatment is hampered by toxicity and resistant parasites. Furthermore, as human, and canine VL causes immunosuppression, the combination of drugs with immunostimulatory agents is interesting to upregulate the immunity, reducing side-effects, improving treatment approaches against disease. Herein, we assessed the immunochemotherapy using miltefosine along with a vaccine formulated by Leishmania braziliensis antigens + saponin + monophosphoryl lipid-A (LBSapMPL) in L. infantum-infected hamsters. Two months after infection, the animals received treatments, and after 15 days they were evaluated for the treatment effect. The potential anti-Leishmania effect of miltefosine + LBSapMPL-vaccine was revealed by a specific immune response activation reflecting in control of spleen parasitism using half the miltefosine treatment time. The treated animals also showed an increase of total and T-CD4 splenocytes producing IFN-γ and TNF-α and a decrease of interleukin-10 and anti-Leishmania circulating IgG. In addition, it was demonstrated that the control of spleen parasitism is related to the generation of a protective Th1 immune response. Hence, due to the combinatorial action of miltefosine with LBSapMPL-vaccine in immunostimulating and controlling parasitism, this immunochemotherapy protocol can be an important alternative option against canine and human VL.