Published online by Cambridge University Press: 19 August 2021
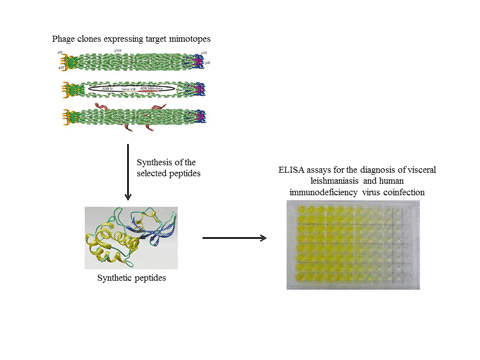
The diagnosis of visceral leishmaniasis (VL) has improved with the search of novel antigens; however, their performance is limited when samples from VL/human immunodeficiency virus (HIV)-coinfected patients are tested. In this context, studies conducted to identify more suitable antigens to detect both VL and VL/HIC coinfection cases should be performed. In the current study, phage display was performed using serum samples from healthy subjects and VL, HIV-infected and VL/HIV-coinfected patients; aiming to identify novel phage-exposed epitopes to be evaluated with this diagnostic purpose. Nine non-repetitive and valid sequences were identified, synthetized and tested as peptides in enzyme-linked immunosorbent assay experiments. Results showed that three (Pep2, Pep3 and Pep4) peptides showed excellent performance to diagnose VL and VL/HIV coinfection, with 100% sensitivity and specificity values. The other peptides showed sensitivity varying from 50.9 to 80.0%, as well as specificity ranging from 60.0 to 95.6%. Pep2, Pep3 and Pep4 also showed a potential prognostic effect, since specific serological reactivity was significantly decreased after patient treatment. Bioinformatics assays indicated that Leishmania trypanothione reductase protein was predicted to contain these three conformational epitopes. In conclusion, data suggest that Pep2, Pep3 and Pep4 could be tested for the diagnosis of VL and VL/HIV coinfection.
Co-senior authors of this work.