Published online by Cambridge University Press: 13 December 2021
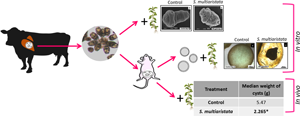
Cystic echinococcosis is a zoonotic disease caused by the larval stage of the parasite Echinococcus granulosus sensu lato. The available anti-parasitic treatment is mostly limited to a continuous administration of albendazole. However, due to its numerous side-effects and efficacy of around 50%, there is a need to find new drugs to improve the treatment for this disease. In the current study, the in vitro and in vivo efficacy of a Stevia multiaristata extract against E. granulosus sensu stricto (s.s.) was demonstrated. Stevia multiaristata extract (100 and 50 μg mL−1) caused a quick viability decrease on protoscoleces which was consistent with the observed tegumental alterations. Loss of turgidity was detected in 95 ± 3.4% of cysts incubated with S. multiaristata extract during 2 days (100 μg mL−1) and the collapse of the germinal layer was observed in 60 ± 9.3% of cysts treated with 100 μg mL−1 of the S. multiaristata extract during 4 days. The half maximal effective concentration value was 69.6 μg mL−1 and the selectivity index for E. granulosus s.s. cysts was 1.9. In this clinical efficacy study, the treatment of infected mice with the S. multiaristata extract (50 mg kg−1) caused a significant decrease in the weight of the cysts compared with the control group. These results coincided with the tissue damage observed in the cysts at the ultrastructural level. In conclusion, we observed high protoscolicidal and cysticidal effects, and significant reduction in the weight of the cysts in experimentally infected mice following treatment with the S. multiaristata extract.