Introduction
The Paleolithic diet (PaleoDiet) is a hunter−gatherer dietary pattern inspired by the consumption of wild foods that were consumed in the Paleolithic age(Reference Whalen, Judd and McCullough1), a period which lasted from 2·8 million years ago until 12 000 BC. Previous studies using isotopes have found that food consumption during this era provided a greater amount of nutrients and energy mainly from large terrestrial herbivores such as horses, pigs, boar and deer(Reference Henry, Brooks and Piperno2), from small and aquatic animals(Reference Henry, Brooks and Piperno2–Reference Richards and Trinkaus6) and from wild plant foods(Reference Bocherens, Hublin and Richards7). However, the specific foods consumed were highly dependent upon the geographical area, the weather and soil conditions(Reference Eaton and Cordain8).
Some studies have hypothesised that the PaleoDiet may reduce the incidence of the metabolic syndrome(Reference Boers, Muskiet and Berkelaar9), type 2 diabetes (T2D)(Reference Frassetto, Schloetter and Mietus-Synder10), CVD(Reference Whalen, Judd and McCullough1) and cancer(Reference Whalen, McCullough and Flanders11). Moreover, several systematic reviews have shown the potential protective effect of the PaleoDiet on cardiovascular risk factors(Reference Ghaedi, Mohammadi and Mohammadi12), on glucose metabolism and insulin homeostasis impairment(Reference Jamka, Kulczyński and Juruć13) and on anthropometric measurements(Reference De Menezes, de Carvalho Sampaio and Carioca14). However, these associated potential benefits are not accepted without controversy, notably because of the restrictions of dairy products, legumes and grains, which generally are considered healthy foods(Reference Fenton and Fenton15). From a public health perspective, this issue is very relevant because the PaleoDiet is becoming increasingly popular, especially among young adults and athletes(Reference Manheimer, van Zuuren and Fedorowicz16).
To the best of our knowledge, no previous review has analysed current scientific evidence considering the definition of the PaleoDiet. In order to offer a realistic approach of the PaleoDiet, it has to balance the, sometimes limited, knowledge about the diet of our ancestors and the need to adapt this to our present food availability and preparation(Reference Truswell17). Therefore, different decisions can be made regarding which foods should be recommended or banned to follow the PaleoDiet and this may have an influence on the health effects associated with this dietary pattern.
In the present comprehensive review, our main objective was to assess PaleoDiet definitions currently published involving its food and nutritional composition and the consistency of these definitions with the ideal hunter−gatherer diet. A secondary aim was to summarise the health effects of the PaleoDiet in the studies that have used these definitions of the PaleoDiet. In addition, we report a new definition of the PaleoDiet based on the most common items used to create previous PaleoDiet definitions and assuming those theoretical criteria that could better feature a diet with available foods during the Paleolithic era.
Methods
Our comprehensive review is based upon a search in PubMed database and a bibliographic search conducted until December 2019. According to our main objective, the inclusion criteria were studies conducted with human subjects, using an interventional or observational design, with an explicit definition of the PaleoDiet and where health-related effects were assessed. The exclusion criteria were narrative or systematic reviews or meta-analyses and articles published in languages other than Spanish or English.
Our search strategy combined the Medical Subject Headings (MeSH) term ‘Paleolithic diet’ and several terms related to the definition, components, recommendations or diet scores amongst others. To expand our search, we added a second search strategy where we combined the term ‘Paleolithic diet’ with a list of foods usually recommended (vegetables, fruits, meat, nuts, eggs), not allowed within the PaleoDiet (legumes, grains, refined grains, salt, additives, dairy products and processed foods) and alcohol. This list of foods is based on anthropological studies of the diet of our ancestors before agriculture started(Reference Challa, Bandlamudi and Uppaluri18).
The flow diagram of the results of this search is shown in Fig. 1. From seventy-five articles initially retrieved, we excluded forty-one narrative reviews and ten systematic reviews(Reference Ghaedi, Mohammadi and Mohammadi12,Reference De Menezes, de Carvalho Sampaio and Carioca14,Reference Manheimer, van Zuuren and Fedorowicz16,Reference Pickworth, Deichert and Corroon19–Reference Carter, Achana and Troughton25) . We finally selected twenty-seven articles after adding four studies from our bibliographic search, which analyse the association between the PaleoDiet and health outcomes.
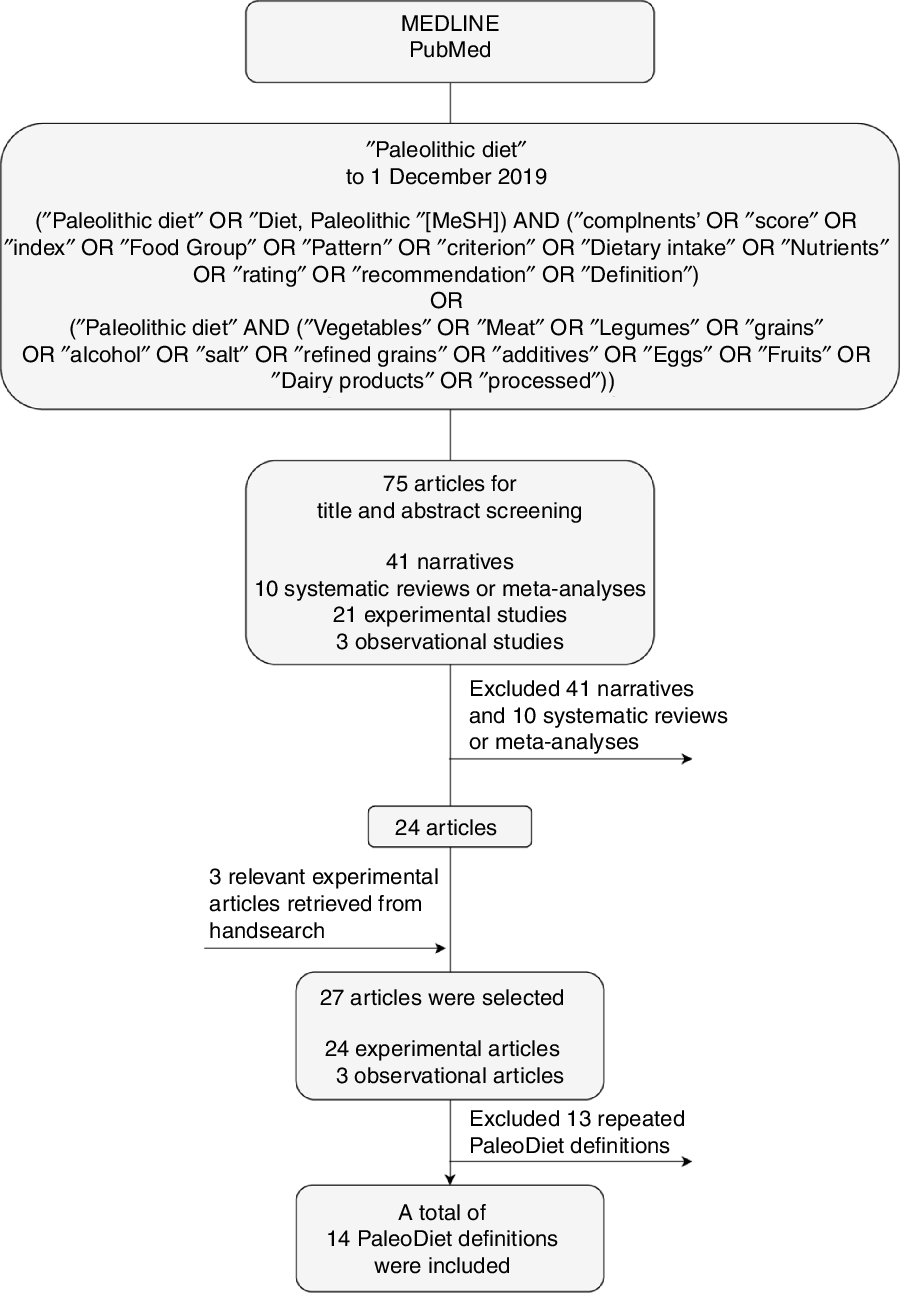
Fig. 1. Search strategy. MeSH, Medical Subject Headings; PaleoDiet, Paleolithic diet.
From these twenty-seven articles we found fourteen different PaleoDiet definitions. One definition was used in seven articles which analysed data from a 2-year intervention study conducted in seventy postmenopausal overweight or obese women(Reference Boraxbekk, Stomby and Ryberg26–Reference Otten, Ryberg and Mellberg32); another definition was used in three articles that used data from a parallel trial conducted in participants with T2D(Reference Stomby, Otten and Ryberg33–Reference Otten, Stomby and Waling35); another three articles presented different results from a cross-over 12-week trial also with T2D participants(Reference Jönsson, Granfeldt and Lindeberg4,Reference Fontes-Villalba, Lindeberg and Granfeldt36,Reference Jönsson, Granfeldt and Ahrén37) ; and another in two studies explored different physiological mechanisms related to the PaleoDiet from a trial conducted in participants with T2D(Reference Frassetto, Shi and Schloetter38,Reference Masharani, Sherchan and Schloetter39) . One common definition was found in three observational studies conducted in the USA(Reference Whalen, Judd and McCullough1,Reference Whalen, McCullough and Flanders11,Reference Whalen, McCullough and Flanders40) . Finally, nine different definitions were used in the other studies(Reference Boers, Muskiet and Berkelaar9,Reference Frassetto, Schloetter and Mietus-Synder10,Reference Bisht, Darling and Grossmann41–Reference Pastore, Brooks and Carbone47) .
The assessment of the PaleoDiet definitions was independently performed by two reviewers (V.d. l. O. and M. R.-C.) and discrepancies were resolved by consensus.
Results
Definitions of the Paleolithic diet
In this section, we provide detailed information about the different definitions of the PaleoDiet patterns previously published. This issue is a relevant matter to better understand why this dietary pattern could be beneficial to prevent chronic diet-related diseases, but also to know if the definition of the PaleoDiet is consistent among previous epidemiological studies.
In this context, Table 1 presents the items used to define fourteen different versions of the PaleoDiet from the twenty-seven studies identified in the present review. We classified as different definitions those reported by Frassetto et al. in 2009(Reference Frassetto, Schloetter and Mietus-Synder10) and 2013(Reference Frassetto, Shi and Schloetter38) because in the latter no component characteristic of the PaleoDiet was provided and we observed different foods that should be prohibited (added sugar and salt). However, these can be differences in the reporting but not in the definition of the PaleoDiet. Similarly, the definition reported by Pastore et al. (Reference Pastore, Brooks and Carbone47) was adapted from the work by Jönsson et al. (Reference Jönsson, Granfeldt and Ahrén37) and therefore these definitions could also be explained by differences in the reporting of the components used in the PaleoDiet definition.
Table 1. List of Paleolithic diet definitions
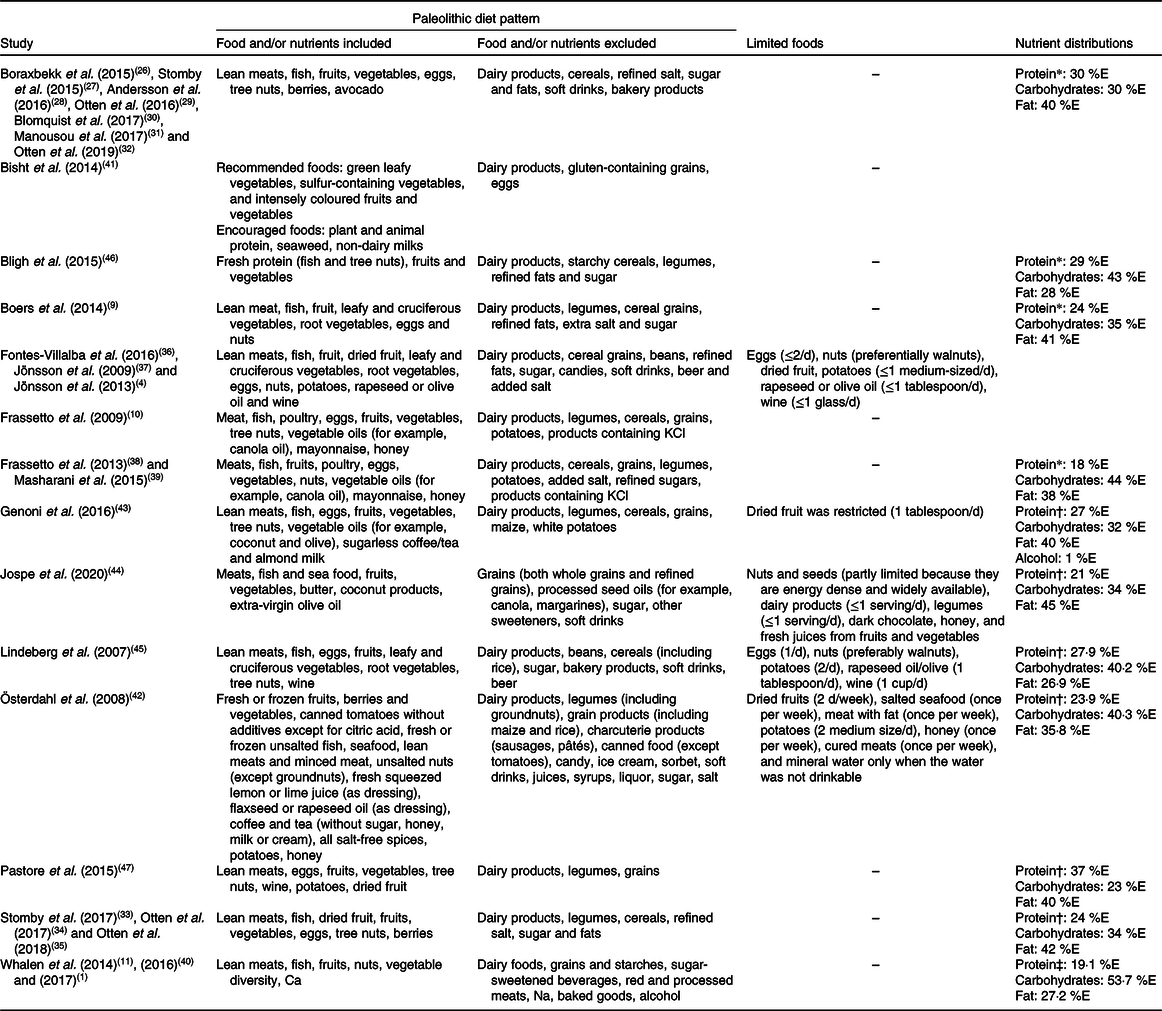
%E, percentage of total energy intake.
* A priori-defined nutrient distribution.
† Reported after the intervention.
‡ Reported highest quintile.
Table 1 shows that most definitions included lean meat, eggs, fish, fruits, vegetables and nuts. Some definitions made a distinction between recommended, allowed or limited foods and some of them restricted the type of foods such as vegetables (i.e. cruciferous) or fruits (i.e. berries or coloured fruits). A common aspect in most definitions is the exclusion of grown foods from agriculture such as cereals or legumes, dairy products as well as added salt, sugar, processed foods and sweets. However, some contradictions were also found concerning the criteria used for some foods, such as potatoes(Reference Blomquist, Chorell and Ryberg30,Reference Manousou, Stål and Larsson31,Reference Österdahl, Kocturk and Koochek42,Reference Lindeberg, Jönsson and Granfeldt45,Reference Pastore, Brooks and Carbone47) or alcohol(Reference Lindeberg, Jönsson and Granfeldt45,Reference Pastore, Brooks and Carbone47) , because some authors decided to exclude these foods or drinks as part of the PaleoDiet score definition. Regarding alcohol, one glass or less of wine was allowed in the PaleoDiet although it was not part of the PaleoDiet definition(Reference Blomquist, Chorell and Ryberg30,Reference Manousou, Stål and Larsson31,Reference Österdahl, Kocturk and Koochek42,Reference Lindeberg, Jönsson and Granfeldt45,Reference Pastore, Brooks and Carbone47) . Conversely, there are also some items, such as mayonnaise(Reference Frassetto, Schloetter and Mietus-Synder10,Reference Masharani, Sherchan and Schloetter39) , sugar-free tea(Reference Genoni, Lyons-Wall and Lo43), coffee(Reference Österdahl, Kocturk and Koochek42,Reference Genoni, Lyons-Wall and Lo43) , butter(Reference Jospe, Roy and Brown44) or coconut products(Reference Jospe, Roy and Brown44), that have been included in some of the definitions of the PaleoDiet, which should be a matter of debate.
Table 1 also shows the macronutrient distribution reported in some studies. Four studies(Reference Boers, Muskiet and Berkelaar9,Reference Boraxbekk, Stomby and Ryberg26,Reference Frassetto, Shi and Schloetter38,Reference Bligh, Godsland and Frost46) presented the a priori distribution of macronutrients according to the definition of the PaleoDiet and seven studies(Reference Whalen, McCullough and Flanders11,Reference Stomby, Otten and Ryberg33,Reference Österdahl, Kocturk and Koochek42–Reference Lindeberg, Jönsson and Granfeldt45,Reference Pastore, Brooks and Carbone47) showed the macronutrient distribution reported by participants as a measure of adherence to the PaleoDiet. The range for protein, carbohydrate and total fat intake was 18–30, 26–44 and 26·9–58 %, respectively.
We have quantified the frequency of inclusion of foods considered characteristic of the PaleoDiet and those that should be excluded according to each of the fourteen PaleoDiet definitions. Tables 2 and 3 show thirty-nine food items in total included at least in one of the definitions of the PaleoDiet score: nineteen food items were positively considered as characteristic of this dietary pattern, while twenty food items were defined as items that should be excluded from the PaleoDiet.
Table 2. Frequency of foods considered as positive characteristics in Paleolithic diet definitions
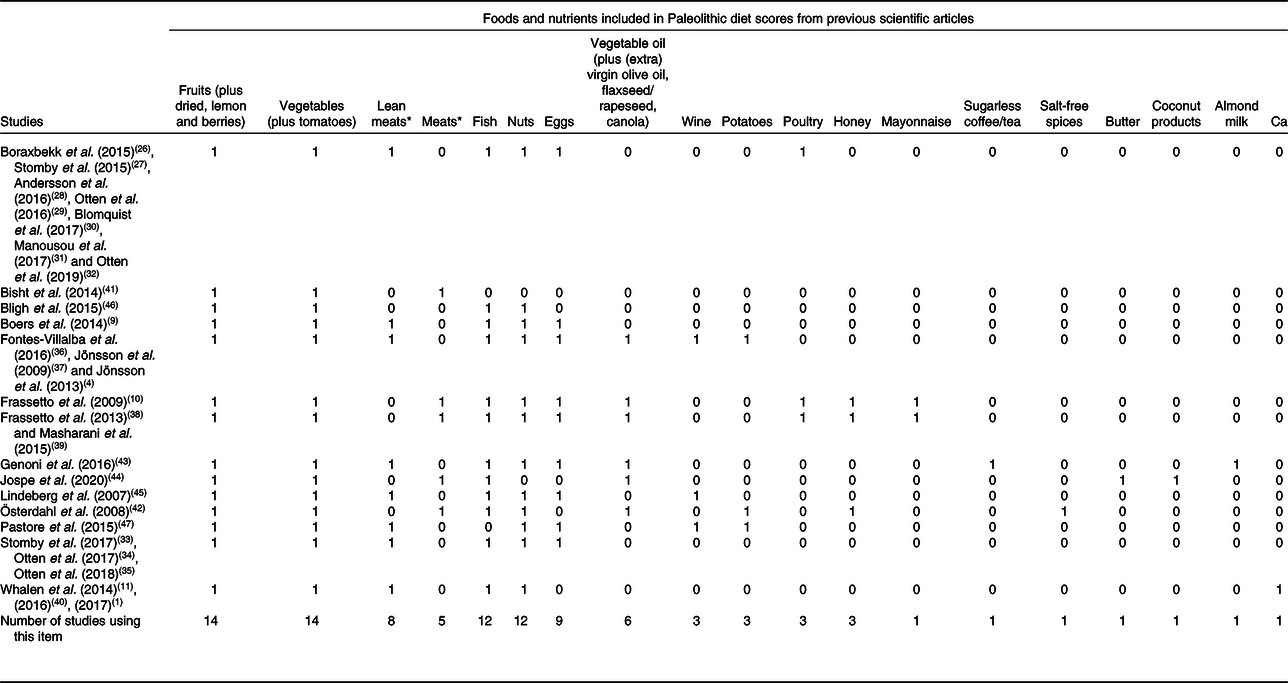
* Thirteen definitions included lean meat or any meat.
Table 3. Frequency of foods considered as negative characteristics in Paleolithic diet definitions
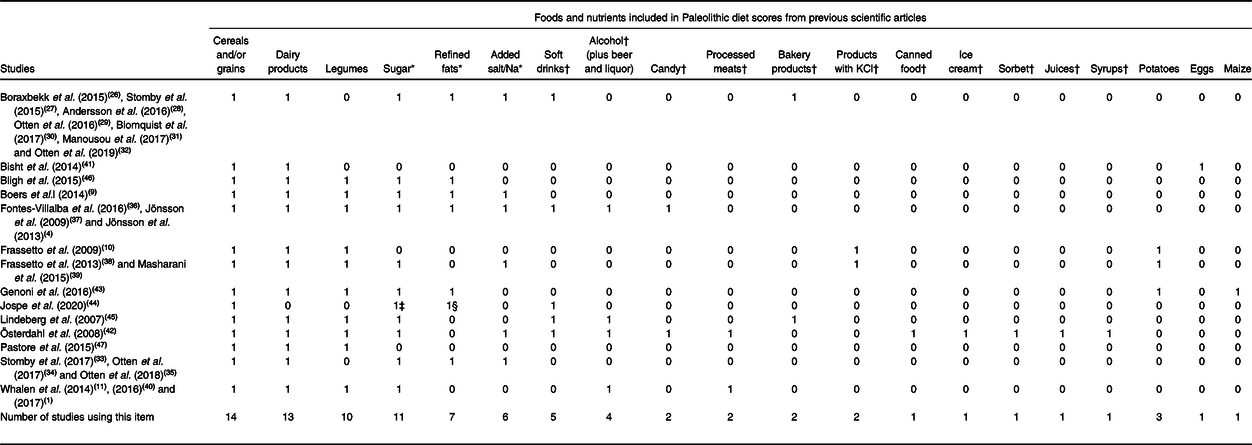
* Culinary ingredients (added sugar, salt/Na, or/and refined fats). Eleven studies included at least one culinary ingredient.
† Processed and ultra-processed foods. Five studies included at least one processed or ultra-processed food.
‡ Only Jospe et al. (2020)(Reference Jospe, Roy and Brown44) rated ‘sweeteners’.
§ Only Jospe et al. (2020)(Reference Jospe, Roy and Brown44) rated ‘processed seed oils’.
As shown in Table 2, the most frequently used items were fruits, vegetables, meats (red meats and lean meats), fish, tree nuts and eggs, since they were included in at least eight out of fourteen definitions. Vegetable oils were included in six definitions although we have grouped in this category different oil sources including olive, flaxseed, rapeseed and canola. The least mentioned items were mainly specific foods such as poultry, honey, wine, potatoes, mayonnaise, sugarless coffee or tea, salt-free spices, butter, coconut products, almond milk and Ca. Some foods such as mayonnaise were included because they contained nutrient characteristics of the PaleoDiet(Reference Frassetto, Schloetter and Mietus-Synder10,Reference Frassetto, Shi and Schloetter38,Reference Masharani, Sherchan and Schloetter39) .
Table 3 shows the twenty items that were defined as foods that should not be included as part of the PaleoDiet definition. The following items were included in at least eight out of fourteen definitions of the PaleoDiet: cereals and grains, dairy products, legumes, sugar and refined fats. Added salt was another culinary ingredient included in six studies. We grouped other foods in the category of processed or ultra-processed foods including soft drinks, alcohol (beer and liquor), candies, processed meats, products with KCl, bakery products, ‘charcuterie’ products, canned food, ice cream, sorbet, juices, syrups, eggs and processed seed oils. One definition of the PaleoDiet excluded specifically non-lean red meat(Reference Whalen, McCullough and Flanders11). Contrary to most definitions, eggs were excluded in a modified PaleoDiet used in a study on fatigue and multiple sclerosis because its elimination could improve the recovery of these patients(Reference Bisht, Darling and Grossmann41).
All the PaleoDiet definitions, except one proposed by Whalen et al. (Reference Whalen, McCullough and Flanders11), were used in clinical trials in which a list of encouraged/recommended and limited/prohibited foods was provided to participants as part of the nutritional intervention. Change in macronutrient intake was generally used to measure adherence to the PaleoDiet in participants assigned to this group intervention. Thus, an increase in protein and fat intake, especially MUFA and PUFA, and a decrease in carbohydrate intake were considered as criteria to measure adherence to the PaleoDiet. Some studies also checked an increase in other specific nutrients such as fibre, K, or n-3 fatty acids as well as a decrease in Na or n-6 fatty acid intake.
Only the definition by Whalen et al. (Reference Whalen, Judd and McCullough1,Reference Whalen, McCullough and Flanders11,Reference Whalen, McCullough and Flanders40) used a score to quantify the PaleoDiet. This definition was used in three different studies(Reference Whalen, Judd and McCullough1,Reference Whalen, McCullough and Flanders11,Reference Whalen, McCullough and Flanders40) . Participants were classified into quintiles according to their intake of seven components positively associated with this dietary pattern (vegetables, fruits, vegetable and fruit diversity, lean meats, fish, nuts and Ca) and seven items negatively associated with the PaleoDiet (non-lean red meats/processed meats, Na, dairy foods, grains and starches, baked goods, sugar-sweetened beverages, and alcohol). Thus, the range of possible scores was 14 to 70 points.
In summary, a great although not full agreement was found among the PaleoDiet definitions regarding the basic foods that should be included (vegetables, fruits, meat (preferably lean meat), fish, nuts and eggs) and excluded (grains/cereals, dairy products, legumes, added sugar, salt and refined fats). We identified a high heterogeneity in the criteria applied to define a PaleoDiet pattern regarding concrete foods that have ‘positive characteristics’ of this dietary pattern, such as honey or coconut products, as well as ‘negative characteristics’ such as canned food or ice cream. Moreover, no quantitative score was calculated in all definitions except in the one used in the studies by Whalen et al. (Reference Whalen, Judd and McCullough1,Reference Whalen, McCullough and Flanders11,Reference Whalen, McCullough and Flanders40) .
Definitions of the Paleolithic diet in the context of the anthropological studies
The PaleoDiet is different from other dietary patterns because it is based on anthropological science(Reference Chang and Nowell48). For this reason, we explored the bibliography used in the studies included in the present review to assess how the characteristic components of the PaleoDiet were justified. Four definitions of the PaleoDiet(Reference Whalen, McCullough and Flanders11,Reference Jönsson, Granfeldt and Ahrén37,Reference Lindeberg, Jönsson and Granfeldt45,Reference Pastore, Brooks and Carbone47) described in Table 1 referenced the study by Eaton et al. (Reference Eaton, Cordain and Lindeberg49) where the paradigm of evolutionary health promotion was defended. Eaton & Konner(Reference Eaton and Konner50) published one seminal article in 1985 proposing the PaleoDiet as an alternative to Western diets which are associated with higher incidence of chronic diseases. Then, 25 years later these authors revisited the PaleoDiet definition and they stated that there was a wide agreement to support that, compared with Western diets, the PaleoDiet was higher in total energy intake but lower energy density, higher in protein, lower in carbohydrate and similar in total fat (although lower n-6:n-3 ratio)(Reference Konner and Eaton51). Thus, the estimated percentage of daily energy in the PaleoDiet is 35–40 % for carbohydrates, 25–30 % for proteins and 20–35 % for total fats(Reference Konner and Eaton51). In some studies that showed the macronutrient percentage distribution, lower protein intake and higher fat intake were reported (Table 1).
The study by Boers et al. (Reference Boers, Muskiet and Berkelaar9) supported their PaleoDiet definition according to an analysis of ethnographic studies which estimated that most hunter–gatherers consumed high amounts of animal foods (45–65 % of energy)(Reference Cordain, Miller and Eaton52). These authors also referred to another study estimating that the PaleoDiet contained higher protein and n-3 fatty acid intakes(Reference Kuipers, Luxwolda and Janneke Dijck-Brouwer53). In fact, Boers et al. (Reference Boers, Muskiet and Berkelaar9) defined a PaleoDiet with 24 % of total energy coming from proteins and 82 % of total protein having an animal source. Finally, Genoni et al. (Reference Genoni, Lyons-Wall and Lo43) used a book by Cordain as a reference to define the PaleoDiet(Reference Cordain54).
Paleolithic diet and health outcomes
We selected twenty-seven articles(Reference Whalen, Judd and McCullough1,Reference Jönsson, Granfeldt and Lindeberg4,Reference Boers, Muskiet and Berkelaar9–Reference Whalen, McCullough and Flanders11,Reference Boraxbekk, Stomby and Ryberg26–Reference Pastore, Brooks and Carbone47) published between 2007 and 2020. Table 4 shows the design, population, comparison group, main outcomes and results from twenty-four articles using an interventional design(Reference Jönsson, Granfeldt and Lindeberg4,Reference Boers, Muskiet and Berkelaar9,Reference Frassetto, Schloetter and Mietus-Synder10,Reference Boraxbekk, Stomby and Ryberg26–Reference Masharani, Sherchan and Schloetter39,Reference Bisht, Darling and Grossmann41–Reference Pastore, Brooks and Carbone47) and three using an observational epidemiological design(Reference Whalen, Judd and McCullough1,Reference Whalen, McCullough and Flanders11,Reference Whalen, McCullough and Flanders40) .
Table 4. Main characteristics of the studies showing an association between the Paleolithic diet (PaleoDiet) and different outcomes
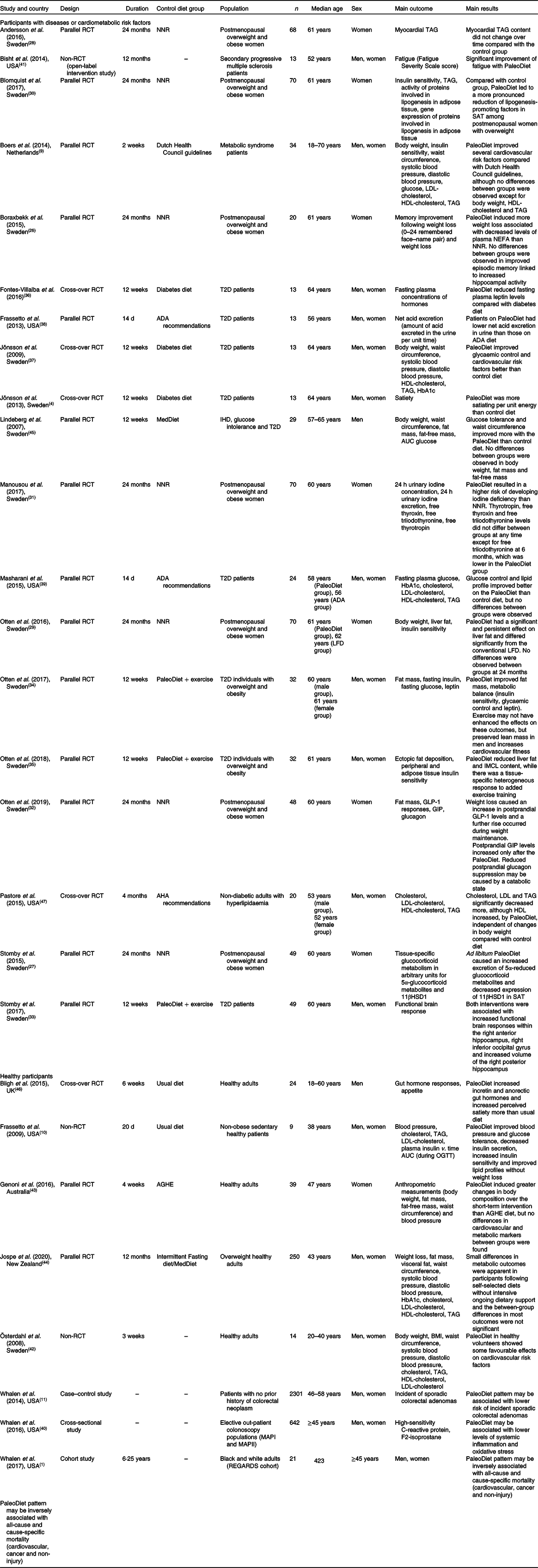
11βHSD1, 11β hydroxysteroid dehydrogenase type 1; ADA, American Diabetes Association; AGHE, Australian Guide to Healthy Eating; AHA, American Heart Association; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; IMCL, intramyocellular lipid; LFD, low-fat diet; MAPI, Markers of Adenomatous Polyps I; MAPII, Markers of Adenomatous Polyps II; MedDiet, Mediterranean diet; NNR, Nordic Nutrition Recommendations; OGTT, oral glucose tolerance test; RCT, randomised controlled trial; REGARDS, REasons for Geographic and Racial Differences in Stroke; SAT, subcutaneous adipose tissue; T2D, type 2 diabetes.
Some of these twenty-seven studies analysed data from the same trial and, therefore, the descriptive characteristics shown in Table 4 are similar in some articles except that in some of them the total number and duration is not the same and each article is analysing different main outcomes. Among twenty-seven articles, nineteen of them included participants with some cardiometabolic disease such as T2D(Reference Jönsson, Granfeldt and Lindeberg4,Reference Otten, Stomby and Waling34–Reference Masharani, Sherchan and Schloetter39,Reference Lindeberg, Jönsson and Granfeldt45) , overweight/obesity(Reference Boers, Muskiet and Berkelaar9,Reference Boraxbekk, Stomby and Ryberg26–Reference Manousou, Stål and Larsson31) and hyperlipidaemia(Reference Pastore, Brooks and Carbone47), and the main outcomes were related to intermediate endpoints such as body weight, glucose, insulin resistance, glycosylated Hb or lipid profile. Only one study analysed the risk of hard endpoints such as cancer or CVD mortality with large follow-up(Reference Whalen, Judd and McCullough1).
Anthropometrics measurements and body composition
The results of long-term (up to 6 months) and short-term (14 d) PaleoDiet interventions on body weight, body fat and waist circumference suggest that this dietary pattern may have a positive impact. Table 5 shows the results comparing a PaleoDiet intervention with different control diets.
Table 5. Main results of the studies analysing the effect of the Paleolithic diet (PaleoDiet) on different outcomes
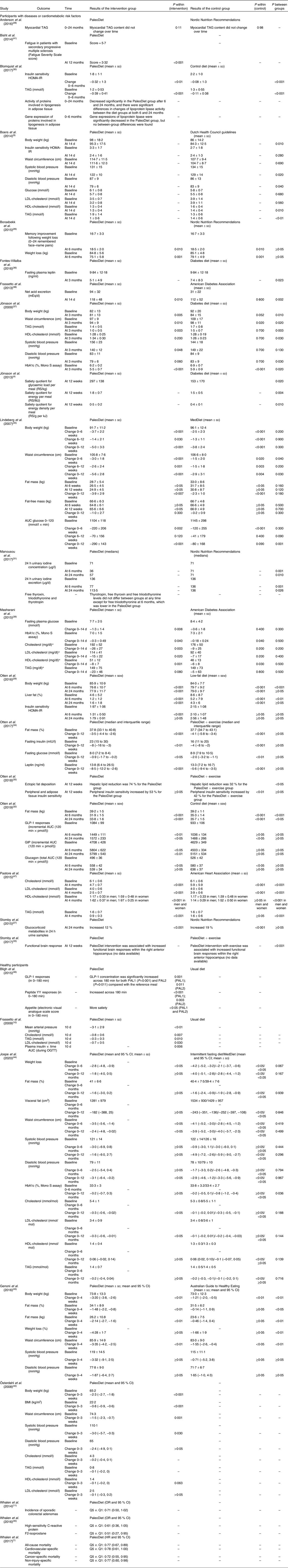
GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; HOMA-IR, homeostatic model assessment of insulin resistance; HR, hazard ratio; MedDiet, Mediterranean diet; OGTT, oral glucose tolerance test; PAL1, Palaeolithic-type meal 1; PAL2, Palaeolithic-type meal 2; Q1, quintile 1; Q5, quintile 5; RS, Rating Scale units.
* To convert cholestereol in mg/dl to mmol/l, multiply by 0·0259. To convert TAG in mg/dl to mmol/l, multiply by 0·0113.
Eight studies assessed the effect of the PaleoDiet on body weight or weight loss and their results suggested that this diet may have a positive effect on the comparison of these variables in participants before and after following this diet(Reference Boers, Muskiet and Berkelaar9,Reference Boraxbekk, Stomby and Ryberg26,Reference Otten, Mellberg and Ryberg29,Reference Jönsson, Granfeldt and Ahrén37,Reference Österdahl, Kocturk and Koochek42–Reference Lindeberg, Jönsson and Granfeldt45) . However, no significant differences were observed when the PaleoDiet was compared with the Nordic Nutrition Recommendations after 6 months(Reference Boraxbekk, Stomby and Ryberg26), with the Mediterranean diet(Reference Lindeberg, Jönsson and Granfeldt45) throughout the follow-up, with a low-fat diet after 24 months of follow-up(Reference Otten, Mellberg and Ryberg29) or in comparison with intermittent fasting or the Mediterranean diet(Reference Jospe, Roy and Brown44).
Several studies(Reference Otten, Ryberg and Mellberg32,Reference Otten, Stomby and Waling34,Reference Genoni, Lyons-Wall and Lo43–Reference Lindeberg, Jönsson and Granfeldt45) suggested a fat-mass reduction among participants within the PaleoDiet, except one study conducted with healthy participants with overweight(Reference Jospe, Roy and Brown44). No significant differences were observed between the PaleoDiet and control groups except in a trial conducted with obese postmenopausal women(Reference Otten, Ryberg and Mellberg32) and in a 4-week trial with Australian healthy overweight women(Reference Genoni, Lyons-Wall and Lo43).
Six studies analysed the effect of the PaleoDiet on waist circumference, an indicator of abdominal obesity. Three trials found a significant waist circumference reduction in the PaleoDiet group compared with the Mediterranean diet(Reference Lindeberg, Jönsson and Granfeldt45), the Australian Guide to Healthy Eating(Reference Genoni, Lyons-Wall and Lo43) or the diabetes diet(Reference Jönsson, Granfeldt and Ahrén37). Other two trials found significant reductions within participants when compared before and after exposure to the PaleoDiet(Reference Österdahl, Kocturk and Koochek42,Reference Jospe, Roy and Brown44) . Boers et al. (Reference Boers, Muskiet and Berkelaar9) found a similar effect in the PaleoDiet and control groups.
Finally, studies on visceral fat deposition reported inconsistent findings(Reference Otten, Mellberg and Ryberg29,Reference Otten, Stomby and Waling35,Reference Jospe, Roy and Brown44) . Otten et al. (Reference Otten, Mellberg and Ryberg29) observed a significantly less marked increase in fatty liver in participants consuming the PaleoDiet compared with a low-fat diet after 6 months of intervention. In another study from the same research group(Reference Otten, Stomby and Waling35), a more pronounced reduction in liver fat deposition in the PaleoDiet group was found compared with a group where PaleoDiet and physical exercise recommendations were implemented. Another study found that visceral fat decreased more in those who followed the control diet (MedDiet or intermittent fasting diet) than in the PaleoDiet group although there were no significant differences between groups in the long term(Reference Jospe, Roy and Brown44).
Cardiometabolic risk factors
Total cholesterol, LDL- and HDL-cholesterol, TAG and blood pressure were the most common cardiometabolic risk factors analysed in the studies included in the present review (Table 5).
A significant total blood cholesterol reduction was observed in five studies(Reference Frassetto, Schloetter and Mietus-Synder10,Reference Masharani, Sherchan and Schloetter39,Reference Österdahl, Kocturk and Koochek42,Reference Jospe, Roy and Brown44,Reference Pastore, Brooks and Carbone47) . Pastore et al. (2015)(Reference Pastore, Brooks and Carbone47) observed significant differences between the PaleoDiet group and control group following American Heart Association recommendations, but no difference was observed in other studies when the PaleoDiet was compared with American Diabetes Association (ADA) recommendations in a short-term study(Reference Masharani, Sherchan and Schloetter39) and when it was compared with intermittent fasting or the Mediterranean diet after 12 months of follow-up(Reference Jospe, Roy and Brown44).
LDL-cholesterol was assessed in six studies(Reference Boers, Muskiet and Berkelaar9,Reference Frassetto, Schloetter and Mietus-Synder10,Reference Masharani, Sherchan and Schloetter39,Reference Österdahl, Kocturk and Koochek42,Reference Jospe, Roy and Brown44,Reference Pastore, Brooks and Carbone47) . These studies suggested a reduction in LDL blood concentrations in the PaleoDiet group, although only a significant difference between this diet and a control group was observed in Pastore et al. (2015)(Reference Pastore, Brooks and Carbone47).
The effect of the PaleoDiet on HDL-cholesterol levels compared with different control diets was evaluated in six studies(Reference Boers, Muskiet and Berkelaar9,Reference Jönsson, Granfeldt and Ahrén37,Reference Masharani, Sherchan and Schloetter39,Reference Österdahl, Kocturk and Koochek42,Reference Jospe, Roy and Brown44,Reference Pastore, Brooks and Carbone47) , but with unclear results. Jönsson et al. (Reference Jönsson, Granfeldt and Ahrén37) and Pastore et al. (Reference Pastore, Brooks and Carbone47) observed a significant increment in HDL-cholesterol levels in the PaleoDiet group, while a null effect with a PaleoDiet intervention was observed in the studies of Boers et al. (Reference Boers, Muskiet and Berkelaar9) and Jospe et al. (2020)(Reference Jospe, Roy and Brown44). A reduction in HDL-cholesterol levels after a short-term PaleoDiet intervention was observed although with no significant differences between the PaleoDiet and control diet following the ADA recommendations(Reference Masharani, Sherchan and Schloetter39). Osterdahl et al. (Reference Österdahl, Kocturk and Koochek42) found a non-significant change in HDL-cholesterol levels after 3 weeks of a PaleoDiet intervention.
Blood TAG levels were analysed in nine studies(Reference Boers, Muskiet and Berkelaar9,Reference Frassetto, Schloetter and Mietus-Synder10,Reference Andersson, Mellberg and Otten28,Reference Blomquist, Chorell and Ryberg30,Reference Jönsson, Granfeldt and Ahrén37,Reference Masharani, Sherchan and Schloetter39,Reference Österdahl, Kocturk and Koochek42,Reference Jospe, Roy and Brown44,Reference Pastore, Brooks and Carbone47) . Most studies showed a higher reduction of TAG levels in the PaleoDiet group compared with the control groups(Reference Boers, Muskiet and Berkelaar9,Reference Frassetto, Schloetter and Mietus-Synder10,Reference Blomquist, Chorell and Ryberg30,Reference Jönsson, Granfeldt and Ahrén37,Reference Österdahl, Kocturk and Koochek42,Reference Pastore, Brooks and Carbone47) . However, no significant difference was observed in another three studies(Reference Andersson, Mellberg and Otten28,Reference Masharani, Sherchan and Schloetter39,Reference Jospe, Roy and Brown44) .
Finally, the PaleoDiet significantly reduced blood pressure over time in five studies(Reference Boers, Muskiet and Berkelaar9,Reference Frassetto, Schloetter and Mietus-Synder10,Reference Jönsson, Granfeldt and Ahrén37,Reference Österdahl, Kocturk and Koochek42,Reference Genoni, Lyons-Wall and Lo43) . One study(Reference Jospe, Roy and Brown44) showed similar results when the PaleoDiet intervention was compared with the Mediterranean diet or the intermittent fasting diet.
Type 2 diabetes markers
The characteristics and the strength of association found in experimental studies that evaluated the effects of diets on T2D risk markers are shown in Table 5.
HbA1c levels were reduced after a PaleoDiet intervention in a short-term study although a non-significant difference was observed in comparison with the ADA recommendations(Reference Masharani, Sherchan and Schloetter39). On the contrary, a higher reduction of HbA1c levels was found in the diabetes diet and Mediterranean diet compared with the PaleoDiet after 3 and 12 months of follow-up, respectively(Reference Jönsson, Granfeldt and Ahrén37,Reference Jospe, Roy and Brown44) .
A short-term reduction in glucose levels was observed related with the PaleoDiet(Reference Boers, Muskiet and Berkelaar9,Reference Otten, Stomby and Waling34,Reference Masharani, Sherchan and Schloetter39) with no significant differences compared with control groups. Lindeberg et al. (Reference Lindeberg, Jönsson and Granfeldt45) observed a higher improvement in glucose tolerance after 12 weeks in the PaleoDiet group compared with the Mediterranean diet group.
Six intervention studies(Reference Boers, Muskiet and Berkelaar9,Reference Frassetto, Schloetter and Mietus-Synder10,Reference Otten, Mellberg and Ryberg29,Reference Blomquist, Chorell and Ryberg30,Reference Otten, Stomby and Waling34,Reference Otten, Stomby and Waling35) compared the effect of a PaleoDiet and other control diets on insulin sensitivity. Several studies did not find significant results(Reference Boers, Muskiet and Berkelaar9,Reference Otten, Mellberg and Ryberg29) . Frassetto et al. (Reference Frassetto, Schloetter and Mietus-Synder10) found a significant reduction in a very short intervention. A significantly higher improvement was observed in the PaleoDiet group compared with a control group after 6 months of follow-up(Reference Blomquist, Chorell and Ryberg30).
Mortality and cancer
One longitudinal cohort study assessed the association between a PaleoDiet score and the risk of all-cause and cause-specific deaths during a median follow-up of 6·25 years(Reference Whalen, Judd and McCullough1). The results suggested that a PaleoDiet may reduce the risk of all-cause mortality and also cardiovascular, cancer and non-injury-specific mortality (Table 5).
The association between colorectal neoplasm and the PaleoDiet was assessed in a case–control study (Table 4)(Reference Whalen, McCullough and Flanders11). This study found a non-significant inverse association in the comparison between the lowest and highest quintile of adherence to a PaleoDiet score after adjusting for other risk factors (Table 5).
Cognitive and brain function
One small pilot study investigated the feasibility of a multimodal intervention with a modified PaleoDiet and its effect on perceived fatigue in patients with secondary multiple sclerosis(Reference Bisht, Darling and Grossmann41). This study showed that a PaleoDiet intervention significantly improved fatigue in these patients after 12 months of follow-up.
Cognitive function was analysed in two studies(Reference Boraxbekk, Stomby and Ryberg26,Reference Stomby, Otten and Ryberg33) . The first study found increased brain activity in postmenopausal obese women following the PaleoDiet and compared with a group following a standard diet (Table 5)(Reference Boraxbekk, Stomby and Ryberg26). Similar results were found in another small-sized study with diabetics which found that both a PaleoDiet and PaleoDiet plus physical exercise could increase some functional brain responses after weight loss and improved insulin sensitivity(Reference Stomby, Otten and Ryberg33).
Mechanisms underlying the effect of the Paleolithic diet
Several studies found in the present review were focused on different mechanisms that could explain the potential beneficial effects of the PaleoDiet(Reference Stomby, Simonyte and Mellberg27,Reference Blomquist, Chorell and Ryberg30,Reference Otten, Ryberg and Mellberg32,Reference Otten, Stomby and Waling34,Reference Fontes-Villalba, Lindeberg and Granfeldt36,Reference Frassetto, Shi and Schloetter38,Reference Whalen, McCullough and Flanders40,Reference Bligh, Godsland and Frost46) .
One parallel and a small cross-over trial investigated PaleoDiet consumption and the influence over the concentrations of glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), leptin and peptide YY (PYY) (Table 5)(Reference Otten, Ryberg and Mellberg32,Reference Bligh, Godsland and Frost46) . An increase in PYY was observed during 0–180 min in the PaleoDiet group compared with the usual diet(Reference Bligh, Godsland and Frost46). Both studies showed a significant increment in GLP-1 and GIP concentration after a PaleoDiet intervention, suggesting that the effect of the PaleoDiet on weight loss and T2D prevention could be mediated through these actions on GLP-1, GIP and PYY.
One small cross-over trial and a parallel trial investigated the effect of the PaleoDiet on fasting plasma leptin levels(Reference Otten, Stomby and Waling34,Reference Fontes-Villalba, Lindeberg and Granfeldt36) . Otten et al. (Reference Otten, Stomby and Waling34) found improvements in leptin concentrations among participants with a PaleoDiet + exercise intervention, but Fontes-Villalba et al. (Reference Fontes-Villalba, Lindeberg and Granfeldt36) did not find differences between diets when comparing the PaleoDiet with the diabetes diet.
In a controlled trial, postmenopausal overweight and obese woman were randomised to eat an ad libitum PaleoDiet-style diet or a control diet for 24 months with the objective to study the gene expression and activity of proteins involved in lipogenesis and lipolysis in adipose tissue(Reference Blomquist, Chorell and Ryberg30). After 6 and 24 months, a significant decrease within the PaleoDiet group and significant differences in changes of lipoprotein lipase activity compared with the control diet were observed. Moreover, the gene expressions of lipoprotein lipase were significantly decreased in the PaleoDiet group, although no between-group differences were found.
One parallel trial analysed glucocorticoid metabolites and urinary excretion in postmenopausal overweight and obese women(Reference Stomby, Simonyte and Mellberg27). A long-term (24 months) weight-loss intervention with a PaleoDiet caused a 12 % increase in the excretion of 5α-reduced glucocorticoid metabolites and decreased expression of 11βHSD1 (11β hydroxysteroid dehydrogenase type 1) in subcutaneous adipose tissue (Table 5).
One intervention study with T2D patients analysed the amount of acid excreted in the urine per unit time following a PaleoDiet compared with nutritional recommendations from the ADA(Reference Frassetto, Shi and Schloetter38). They observed that after a short-term intervention (14 d), the net acid load of the patients on the PaleoDiet was lower than that of those following the nutritional recommendations from the ADA(Reference Frassetto, Shi and Schloetter38).
Finally, in a cross-sectional study of men and women undergoing a colonoscopy, plasma high-sensitivity C-reactive protein and F2-isoprostane concentrations were measured according to levels of adherence to the PaleoDiet(Reference Whalen, McCullough and Flanders40). A lower probability of systemic inflammation and oxidative stress was associated with a higher adherence to the PaleoDiet (Table 5).
In summary, a number of studies have presented different mechanistic insights related to the beneficial effect of the PaleoDiet on cardiometabolic diseases, especially overweight/obesity and T2D.
Micronutrient intake associated with the Paleolithic diet
Several trials provided information about micronutrient intake and the changes observed after PaleoDiet intervention. A number of these studies showed a lower intake of Na after the PaleoDiet intervention(Reference Frassetto, Schloetter and Mietus-Synder10,Reference Otten, Stomby and Waling34,Reference Masharani, Sherchan and Schloetter39,Reference Österdahl, Kocturk and Koochek42–Reference Lindeberg, Jönsson and Granfeldt45) , and higher intake of K(Reference Frassetto, Schloetter and Mietus-Synder10,Reference Masharani, Sherchan and Schloetter39,Reference Österdahl, Kocturk and Koochek42) .
One parallel trial in postmenopausal overweight and obese woman analysed iodine deficiency and free thyroxine (FT4), free triiodothyronine (FT3) and thyrotropin (TSH)(Reference Manousou, Stål and Larsson31). They found that short-term (6 months) PaleoDiet intervention resulted in lower FT3 than the Nordic Nutrition Recommendations diet. Moreover, a long-term PaleoDiet intervention resulted in a higher risk of developing mild iodine deficiency compared with a control diet (Table 5). Genoni et al. (Reference Genoni, Lyons-Wall and Lo43) also observed a lower of iodine in the PaleoDiet group in a 4-week randomised trial.
Jospe et al. (Reference Jospe, Roy and Brown44) found that Ca intake was significantly higher in the Mediterranean diet group compared with the PaleoDiet group after 6 and 12 months of follow up A lower intake of Ca was also found in a 4-week trial with healthy women(Reference Genoni, Lyons-Wall and Lo43) and in a 3-week pilot study with healthy volunteers(Reference Österdahl, Kocturk and Koochek42).
The Paleolithic diet in perspective with other healthy dietary patterns
According to the present review, current evidence suggests that the PaleoDiet may have potential benefits in the prevention of obesity and T2D, although this evidence is limited due to small sample size, the short follow-up of most studies, and it is based on surrogate outcomes. Different studies analysed in the present review compared the PaleoDiet with other healthy diets such as the American Heart Association, ADA, or Nordic Nutrition Recommendations, the Dutch Health Council guidelines, the Mediterranean diet, the diabetes diet or the Australian Guide to Healthy Eating diet. As shown in Table 5, the improvement associated with the PaleoDiet was statistically significant in comparison with some of these dietary patterns regarding body weight(Reference Boers, Muskiet and Berkelaar9,Reference Jönsson, Granfeldt and Ahrén37,Reference Genoni, Lyons-Wall and Lo43) , HBA1c levels and cholesterol levels(Reference Jospe, Roy and Brown44), and satiety(Reference Österdahl, Kocturk and Koochek42). However, in most of these comparisons the follow-up was short (14 d to 4 months). Moreover, non-significant differences were found in other comparisons in different studies(Reference Boers, Muskiet and Berkelaar9,Reference Boraxbekk, Stomby and Ryberg26–Reference Andersson, Mellberg and Otten28,Reference Masharani, Sherchan and Schloetter39,Reference Jospe, Roy and Brown44,Reference Lindeberg, Jönsson and Granfeldt45) . Of course, these negative results cannot be interpreted as a demonstration of equivalence between these dietary patterns since the sample size is small.
The PaleoDiet, similar to other dietary patterns, measures the overall and synergistic effect of all its components. The potential beneficial effects of the PaleoDiet could be attributed to a high consumption of fruits, vegetables, fish and nuts, which are important sources of fibre, MUFA and PUFA, as well as to a very limited or no consumption of processed and ultra-processed food, and limited intake of added sugar, salt and refined oils.
The PaleoDiet is characterised by relatively high protein intake and high consumption of meat, especially lean meat. Most studies included both red and white meat as part of this diet, although with preference for lean meats. However, getting the difference between lean and non-lean meat to the general population can be tricky. Moreover, the nutrient composition of meat available today is probably different from meat consumed in the Paleolithic era, which was low in saturated fats and higher in n-3 fatty acids, particularly α-linolenic acid, as animals would have been free range(Reference Cordain, Miller and Eaton52). In fact, the consumption of animal protein (especially red meat) during the Paleolithic age may conflict with current nutritional guidelines and studies supporting the reduction of meat intake for both health and environmental reasons. Different studies have already shown the increased risk of cancer with a higher consumption of red and ultra-processed meats(Reference Inoue-Choi, Sinha and Gierach55–Reference de Vries, Quintero and Henríquez-Mendoza59). However, since the PaleoDiet supports the consumption of non-processed meats, more studies are needed to explore the relevance of the quality of protein intake, such as for example the healthy v. the unhealthy sources of protein, beyond the quantity of protein, that would be acceptable for the general population(60). In any case, a higher intake of non-processed meat could be associated with an increased cost and it would not be feasible to recommend this diet for the general population(Reference Genoni, Lo and Lyons-Wall61), and also for the environmental consequences of animal food consumption(Reference Machovina, Feeley and Ripple62).
The recommendation to exclude legumes and whole grains in the PaleoDiet is another source of controversy if compared with recommendations from other healthy diets. Some studies have shown the beneficial effects of legumes, such as risk reduction of IHD(Reference Afshin, Micha and Khatibzadeh63), LDL-cholesterol(Reference Ha, Sievenpiper and de Souza64), systolic blood pressure(Reference Jayalath, de Souza and Sievenpiper65), body fat(Reference Onakpoya, Aldaas and Terry66), and the reduction of oxidative stress, pro-inflammatory marker C-reactive protein and cholesterol levels(Reference Crujeiras, Parra and Abete67,Reference Hermsdorff, Zulet and Abete68) . Moreover, the consumption of legumes is beneficial for environment sustainability because they are N-fixing and therefore they increase soil fertility(69).
The promotion of a relatively high-protein diet may have negative consequences for a long-term adherence of the PaleoDiet. A recent study has shown that total protein intake is quite stable in many countries: about 16 % of total energy, independent of lifestyle and demographic factors(Reference Lieberman, Fulgoni and Agarwal70). According to the ‘protein leverage theory’, humans and other animals avoid high- and low-protein diets and they strongly regulate protein intake in comparison with other macronutrients(Reference Simpson and Raubenheimer71). This fact may help to understand the low level of adherence when free-living humans are advised to change their macronutrient intake, especially protein intake. In fact, compliance with the PaleoDiet can be difficult, especially in the long term(Reference Genoni, Lyons-Wall and Lo43).
Finally, restriction of some foods within the PaleoDiet could determine the adequate intake of some specific micronutrients. According to the definition of the PaleoDiet, this diet could provide the recommended daily intake of all micronutrients(Reference Eaton and Nelson72,Reference Eaton and Eaton73) . In fact, one positive aspect of this restriction is the lower intake of Na and higher intake of K in comparison with other diets. However, a lower intake of iodine could be related to the restriction of iodised table salt and dairy products(Reference Niwattisaiwong, Burman and Li-Ng74) and the restriction of dairy products could also explain the lower intake of Ca(Reference Metzgar, Rideout and Fontes-Villalba75,Reference Hochberg and Hochberg76) .
Proposal of the Paleolithic diet score
In the present review we found that only one definition proposed a score to quantify adherence to the PaleoDiet(Reference Whalen, McCullough and Flanders11). As we have described before, these authors scored PaleoDiet adherence with fourteen items and participants were classified into quintiles according to these items. Among the positive items, two of them were probably based on health reasons but not as a characteristic of the original PaleoDiet. One item was fruit and vegetable diversity, and another was the intake of Ca from sources other than dairy products. On the one hand, the PaleoDiet was dependent of the geographical, seasonal, weather and soil conditions and therefore in most cases it would be not possible to achieve this diversity. On the other hand, in Whalen’s score(Reference Whalen, Judd and McCullough1,Reference Whalen, McCullough and Flanders11,Reference Whalen, McCullough and Flanders40) , Ca intake was indirectly measured in the item on fruit and vegetable intake and the item on fruit and vegetable diversity. Moreover, Ca intake was calculated as residuals from the linear regression of total Ca intake and this also included Ca supplements. The score by Whalen et al. (Reference Whalen, Judd and McCullough1,Reference Whalen, McCullough and Flanders11,Reference Whalen, McCullough and Flanders40) only included beef meet as red lean meat (plus skinless chicken and turkey) and all non-lean red meat was excluded. However, the general opinion is that any red lean meat was characteristic of the diet among hunter–gatherer societies(Reference Mann77). Finally, in this score only sugar-sweetened beverages and baked goods were used as sources of added sugar. In our score described below, we included an item such as processed and ultra-processed foods (that includes other foods that are not sweet but with added sugar and salt) and another item to refer to the culinary ingredients (added sugar, salt and refined fats).
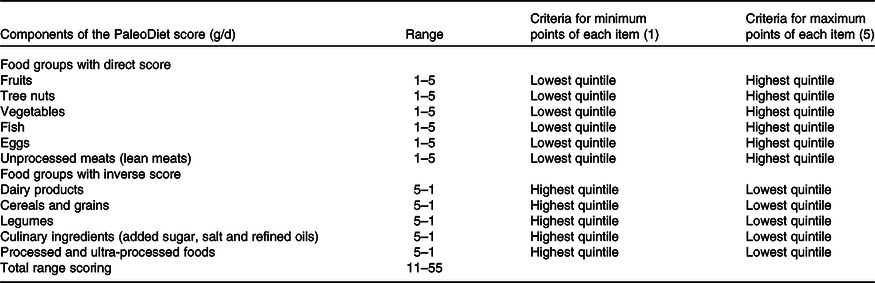
We propose a new definition of the PaleoDiet based on those foods that were most frequently used in the definitions we have identified (items found in ≥50 % of the PaleoDiet definitions), and also considering foods which are consistent with the theoretical definition of the PaleoDiet. As anthropologists have described the PaleoDiet as the diet existing before the development of agriculture(Reference Eaton and Konner50), foods from wild animals and plants were mostly accounted for in our new definition. Also, a numeric score was calculated in order to measure adherence to this dietary pattern.
The new PaleoDiet score that we promote encompasses eleven items: six items refer to foods that should be part of a PaleoDiet: fruits, nuts, fish, vegetables, eggs and meats (lean meats); and another five items refer to dietary variables that should be excluded from the PaleoDiet definition: cereals and grains, dairy products, legumes, culinary ingredients (added sugar, salt and refined oils) and ultra-processed foods (Table 6). We defined a score following a similar approach applied in other dietary patterns such as the Dietary Approaches to Stop Hypertension (DASH)(Reference Fung, Chiuve and McCullough78) score, a Mediterranean diet score(Reference Schröder, Fitó and Estruch79) and the previous PaleoDiet score(Reference Whalen, McCullough and Flanders11). Thus, each of the eleven items was categorised according to quintiles of intake. Those items promoted within the PaleoDiet received a minimum of 1 point for the lowest quintile and a maximum of 5 points for the highest quintile. In contrast, those items negatively associated with the PaleoDiet definition received a maximum of 5 points for the lowest quintile and 1 point for the highest quintile. Finally, we constructed the PaleoDiet score by summing all the values, ranging from 11 (lowest adherence) to 55 (highest adherence). This quantified score could be applied in any observational study once food intake has been derived from FFQ or other method of dietary assessment.
Table 6. Proposal of the new Paleolithic diet (PaleoDiet) score for observational studies
Limitations and strengths
The scoping review
The main limitation of our scoping review is that we have not followed a more thorough methodology such as the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Nevertheless, systematic reviews and meta-analyses are usually focused on specific aims according to one main independent variable (for example, PaleoDiet) and a specific outcome. Our scoping review had a broader perspective since we were focused on the different definitions of the PaleoDiet and secondarily we reviewed the association between these PaleoDiet definitions and several outcomes. Furthermore, our search identified several systematic reviews that included a smaller number of studies related to the PaleoDiet(Reference Ghaedi, Mohammadi and Mohammadi12,Reference De Menezes, de Carvalho Sampaio and Carioca14,Reference Manheimer, van Zuuren and Fedorowicz16) , and our comprehensive review used literature review methods that are specific and thorough to avoid outcome bias(Reference Stratton80). In addition, information extracted from the selected studies in this review was independently performed by two reviewers, who resolved their discrepancies by consensus.
The Paleolithic diet definition
The promotion of the PaleoDiet is based on the concept of evolutionary health promotion(Reference Eaton, Cordain and Lindeberg49). According to this concept, human lifestyles, including diet, have evolved more rapidly than human genetic evolution. Basically, this dissonance may explain the increased morbidity and mortality in our Modern times since the origin of agriculture. According to this, the recovery of the diet of our ancestors should become a new paradigm to reduce the burden of chronic and degenerative diseases associated with our lifestyle.
One important limitation of the PaleoDiet is our partial knowledge about the diet of our ancestors during the Paleolithic age. Anthropological and archeological studies have shed new light about the human diet during this time. For example, an anthropological study about food consumption during this period observed a disparity in relation to the consumption of starch and the heterogeneity of food habits among different populations(Reference Hardy, Buckley and Copeland81). An archeological study has found evidence of cooked starchy plant foods in Africa 170 000 years ago(Reference Wadley, Backwell and D’Errico82). As a consequence, the PaleoDiet will be always defined with some level of uncertainty and without taking into account other characteristics of a hunger–gatherer lifestyle.
Another limitation is the variability of the PaleoDiet due to the availability of food depending on the geographical area and the long period of the Paleolithic era with their respective variations in food consumption and nutritional composition of the diet. Moreover, diets based on ancient civilisations are always dependent on the adaptation of the animal and vegetable species available nowadays. The differences between domestic/supermarket meat v. lean meat and wild plants v. cultivated plants are factors that are difficult to control.
A new Paleolithic diet score
The PaleoDiet was already popular before solid scientific evidence was available. The present review has shown that this dietary pattern may have beneficial effects, especially in relation to cardiometabolic diseases although the evidence is still limited. The definition and adaptation of the PaleoDiet are key aspects as well as the need of a pragmatic and realistic approach to follow a healthy diet(Reference Truswell17). Similar questions are faced with the promotion of traditional diets such as Japanese or Mediterranean diets. However, this is more challenging when we try to restore a diet from ancient times, sometimes with some halo of mysticism such as the Avicennian diet(Reference Naghizadeh, Zargaran and Karimi83).
Both interventional and observational epidemiological studies are needed to support the promotion of any dietary pattern with scientific evidence. The development of diet scores is a common strategy to evaluate the level of adherence and also as simple assessment tools to promote traditional healthy diets(Reference Kanauchi and Kanauchi84,Reference Martínez-González, Gea and Ruiz-Canela85) . We have previously discussed the limitations of the PaleoDiet score found in the present review(Reference Whalen, McCullough and Flanders11). In this review we propose a new score which follows a similar approach to score the PaleoDiet according to the quintiles of adherence to different items. However, we have used a lower number of items and we have modified some of them. Our score is based on a systematic search of previous definitions and the analysis of the theoretical definition of the PaleoDiet. Thus, we have eliminated the use of fruit/vegetable diversity and Ca as components of the PaleoDiet. We have added the use of ultra-processed foods as they have a high content of saturated fats, added salt and sugar as well as low content of fibre(Reference Srour, Fezeu and Kesse-Guyot86,Reference Rico-Campà, Martínez-González and Alvarez-Alvarez87) . Therefore, we consider that ultra-processed foods are representative of the group of foods that should not be part of the PaleoDiet in addition to grains, legumes and dairy products.
One limitation of our score is that no quantitative information is proposed to determine the amount that should be consumed of each food group. Therefore, this score could not be used as a self-administered score to assess adherence to the PaleoDiet. However, we think more epidemiological research is needed before we can promote the PaleoDiet as a healthy diet and also to establish clear recommended cut-offs of each food within this diet. Our proposed score is aimed to measure the PaleoDiet and to quantify the longitudinal relationship of the overall diet with chronic diseases. The use of FFQ is the best approach to capture the usual intake and to explore whether the dietary recommendations are met over time in large populations. The general problem is that these questionnaires are usually based on self-reported information and they have important limitations in capturing the absolute intake of foods and nutrients(Reference Willett88).
Another limitation is the use of population-specific values (quintiles) as cut-off levels for scoring each item of the PaleoDiet. This approach limits the comparison of PaleoDiet scores across populations but when fixed cut-off levels are defined it is possible that some components do not contribute to the overall score because all participants receive the same score(Reference Ocké89). Our approach allows all score components to contribute to the overall PaleoDiet score. An alternative could be the development of a weighted PaleoDiet score, for example with weights according to the burden of disease associated with each component, but more research is needed to use objective weights that can be implemented in different populations(Reference Fransen and Ocké90).
Conclusion
According to our scoping review, scientific evidence suggests beneficial effects of predefined PaleoDiet patterns in lipid markers, obesity, insulin and glucose altered levels, appetite and satiety, and improvements in patients with neurological diseases. However, current evidence about the PaleoDiet is limited because most of the experimental studies are focused on intermediate outcomes, such as body weight or fat mass, instead of hard endpoints such as T2D, CVD or cancer.
We have found that almost all definitions of the PaleoDiet include fruits, nuts, vegetables, fish, eggs and lean meats as characteristic foods of the PaleoDiet. Similarly, dairy products, cereals and grains, legumes and culinary ingredients (added salt, sugar and refined fats) were discouraged foods in most PaleoDiet definitions. However, we have observed a high heterogeneity regarding specific foods that should be added or excluded within the PaleoDiet definition and sometimes with some contradictory criteria such as the exclusion of eggs or potatoes. In this review, we report a simplified operational definition of this dietary pattern and a quantification system mode that could be used in observational studies.
Finally, more nutritional epidemiology research is needed to demonstrate the nutrient adequacy and long-term association of a higher adherence to this PaleoDiet score with the prevention of the most prevalent chronic diseases.
Acknowledgements
The present review received no specific grant from any funding agency, commercial or non-profit sectors.
V. d. l. O. participated in the conception and design of the study, conducted the critical review of current literature and the assessment and wrote the first draft of the manuscript. M. R.-C. and I. Z. developed the concept and design of the present review and supervised the analysis of the results and the writing of the manuscript, J. A. M., S. S., S. C. and M. A. Z. contributed to the design and implementation of the research, to the critical analysis of the results and to the writing of the manuscript. All included authors were involved in the review and subsequent approval of the final version of the manuscript.
There are no conflicts of interest.










