Shift work is extremely frequent in several services and industries, in order to systematise the needs for flexibility of the workforce, necessary to optimise productivity and business competitiveness. Shift work is defined as work primarily outside of normal daytime working hours(Reference Sharifian, Farahani and Pasalar1). In developing countries, this population represents a considerable contingent workforce. Recently, studies showed that overweight and obesity are more prevalent in shift workers than day workers(Reference Di Lorenzo, De Pergola and Zocchetti2, Reference Ostry, Radi and Louie3). In addition, the literature shows that shift workers seem to gain weight more often than those workers submitted to a usual work day(Reference Di Lorenzo, De Pergola and Zocchetti2–Reference Parkes4).
Modern society has come to rely increasingly on 24 h operations in many diverse settings and as many as 20 % of workers in industrialised nations are shift workers(Reference Di Lorenzo, De Pergola and Zocchetti2, Reference Haus and Smolensky5). The circadian rhythm and environmental conditions can become desynchronised in rotating shift workers whose night activity is out of phase with many coupled rhythms due to desynchronisation of the normal phase relationships between biological rhythms within the circadian system.
It is well known that the timing of sleep is under the control of the circadian pacemaker. Humans are a diurnal species; they sleep mostly at night, and they do so at approximately 24 h intervals. If they do not adhere to this general pattern, for instance when working night shifts, they experience the influence of their circadian clock(Reference Beersma and Gordijn6). Also, shift workers may develop sleep disturbances when the relationship between light–dark phase, sleepiness and food intake is desynchronised.
Shift work is associated with various health problems caused by the disturbance of these biological rhythms. In spite of this, overweight and obesity may elicit a series of diseases, resulting in a public health problem. The WHO predicted that about 1·6 billion adults are overweight, and at least 400 million individuals are obese. Therefore, the WHO estimates that there will be 2·3 billion overweight adults in 2015, and that the number of obese individuals will reach 700 million. As BMI increases, it also becomes a greater risk factor for chronic diseases. A high BMI is a risk factor for a series of pathological conditions such as: coronary diseases which are responsible for 17 million deaths per year; diabetes which has become a global epidemic and will increase by 50 % in the next 10 years(7).
The present review summarises data found in the literature about circadian disruption and its relationship to obesity and metabolic disturbances frequently presented by shift workers.
Materials and methods
To introduce the central theme of the present review, we searched for experimental, observational and double-blind controlled randomised clinical trials. We used the MEDLINE (1960–2008) and Cochrane sites in our search strategy to locate the studies. Our language choices were English, Portuguese, Spanish, French and Italian. For this article compilation, we used the following keywords: ‘shift work and obesity’; ‘shift work and overweight’; ‘shift work and BMI’; ‘shift work and metabolic syndrome’. We found 212 articles based on the above search approach and selected ninety-five (Fig. 1). We excluded the articles that were caught in the search strategy at several levels and this was a reason for the reduction of numbers of articles. The inclusion criterion adopted was the obligatory presence in the abstract of the relationship between shift work and metabolic conditions, such as dyslipidaemia, the metabolic syndrome, CVD, alterations of lipidic and glycidic metabolisms, which could be triggered or promoted by shift work.
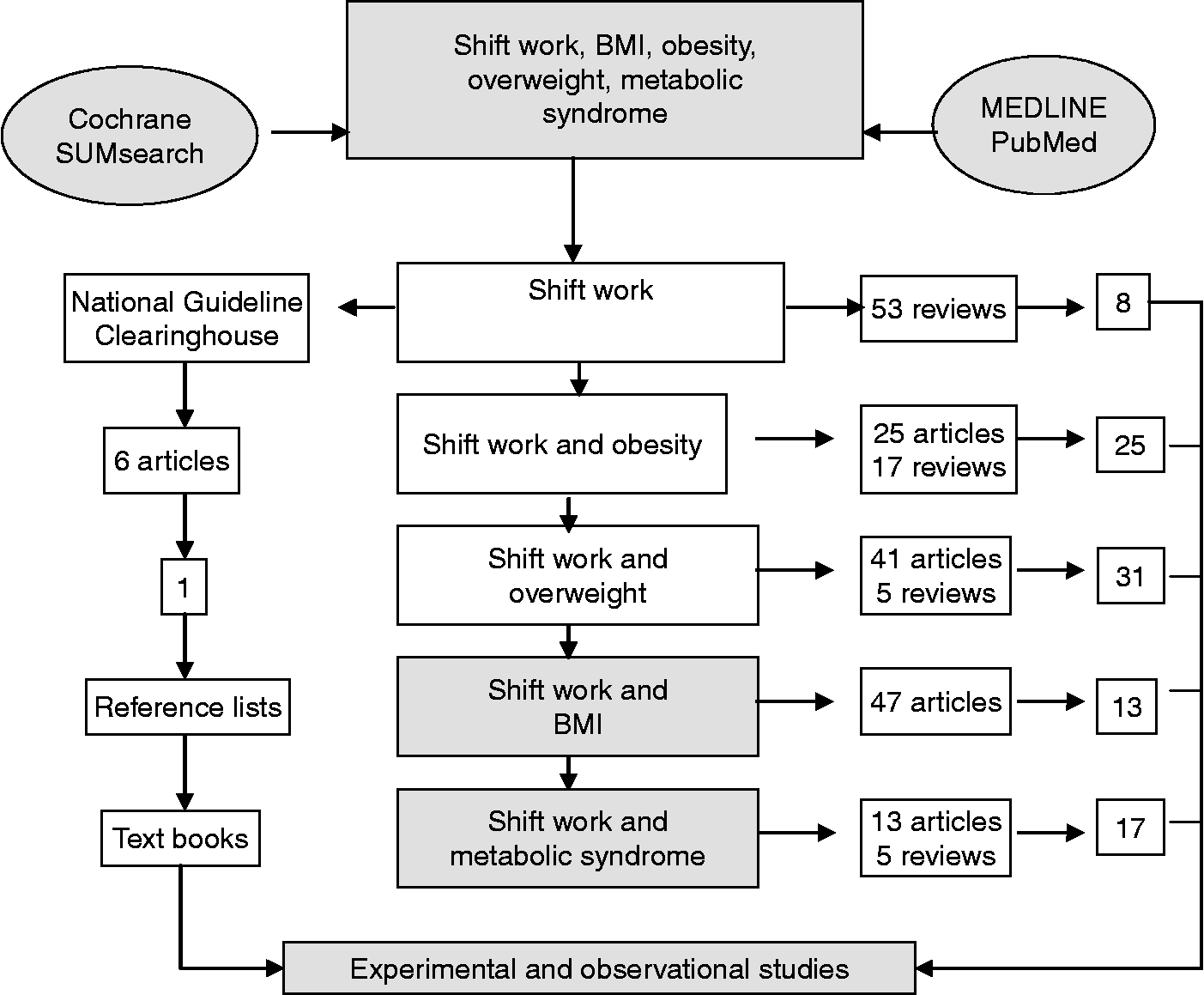
Fig. 1 Steps in the review.
The impact of shift work on anthropometric parameters
Recent studies have shown that overweight and obesity are more prevalent in shift workers than day workers. In addition, the literature shows that shift workers seem to gain weight more often than those workers with the usual work day(Reference Di Lorenzo, De Pergola and Zocchetti2–Reference Parkes4, Reference Morikawa, Nakagawa and Miura8). Shift workers in the selected studies showed variability in overweight and obesity prevalence of about 47·2 and 2·8 %, respectively. Nevertheless, in another study this difference was attributed not only to shift work, but also to the fact that the mean age of day workers was significantly higher(Reference Parkes4).
A study showed overweight and obesity prevalences of 62·4 and 15·7 %, respectively. The authors demonstrated that shift work is associated with BMI independent of age or time in shift work. These data become even more relevant when we consider that this research was conducted in the Mediterranean region(Reference Di Lorenzo, De Pergola and Zocchetti2). The findings are in accordance with the literature, in which shift workers presented higher BMI, although studies concerning the time of exposure to this kind of labour pattern are controversial. In comparing 787 day workers and 787 day–night shift workers, duration of shift work exposure was a highly significant predictor among the day–night shift workers, after controlling for age. The authors concluded that a longer exposure predicts a higher BMI(Reference Parkes4). Also job stress and long working hours seem to contribute to an increase in BMI(Reference Ostry, Radi and Louie3).
A study conducted in Norway covering the period from 1979 to 2001 correlated BMI to shift work(Reference Wilsgaard, Jacobsen and Arnesen9). The association between psychosocial work conditions and BMI seemed to be related to both the effort at work and the time of work, for males. Although the choice of participants was conducted in a random way, this study was limited because weight and height were self-reported and not measured(Reference Ostry, Radi and Louie3). There are studies where obesity was more prevalent in female shift workers than males for all ages studied(Reference Karlsson, Knutsson and Lindahl10). The association between shift work, age and BMI showed that obese subjects (BMI>30 kg/m2) corresponded to 7·5 % of the sample, while the overweight percentage was 47·2 %. The mean BMI differed significantly between day workers and shift workers. Exposure time to shift work was significant, and this finding led us to infer that age as well as shift work years contributed to BMI in an independent and positive way(Reference Parkes4). Shift work duration seems to be positively associated with BMI and waist:hip ratio in male and female populations independent of age, sex, smoking status, physical activity and education(Reference van Amelsvoort, Schouten and Kok11). In Oriental male workers with sedentary functions, shift work, smoking status and marital status were associated with BMI increase. Shift work, excessive alcohol consumption and decrease in physical activity were significantly associated with an increase in waist:hip ratio(Reference Ishizaki, Morikawa and Nakagawa12). Based on these findings, we can conclude that even in Oriental societies overweight and obesity dimensions can reach alarming proportions. On the other hand, Nakamura et al. compared the BMI of shift workers and day workers and could not corroborate these findings(Reference Nakamura, Shimai and Kikuchi13). The reason may be attributed to the origins of this population, since this research was carried out in an Oriental society, where overweight and obesity do not occur in the same proportions seen in Western populations. Interestingly, they found a difference in the waist:hip ratio, where shift workers showed higher measures. It is important to know if shift work in this population could have an influence on fat distribution, rather than BMI, suggesting possible alterations in lipid metabolism.
In comparing 226 nurses and 134 male shift workers from a factory, obesity was found to be 2·8 and 27·9 %, respectively(Reference Ha and Park14). We noticed limitations, such as sample heterogeneity. Differences in sex, socio-economic status and schooling could have influenced obesity. These observations may have contributed to the establishment of weak associations. Nurses aged 30 years or more showed a significant association between time in shift work and waist:hip ratio(Reference Ha and Park14). The reason may be the fact that components such as the wake–sleep cycle, thermogenesis, food intake, and lipid and glucose metabolism are under circadian regulation. This regulation synchronises available and expenditure energy to external changes in the environment, such as the light–dark phase(Reference Gomez-Abellan, Hernandez-Morante and Lujan15). Such rhythms are coordinated by circadian clocks which are intrinsically maintained by molecular mechanisms whose objective is to condition humans to adapt to environmental changes. Circadian clock components have been found in all kinds of mammalian tissues, including adipose tissue. Recent studies suggest a relationship between altered eating habits, wake–sleep pattern, lifestyle and obesity. This relationship may explain the increase in obesity(Reference Bray and Young16). Work schedules that involve the alteration of typical working hours are related to a natural sleep–wake cycle disruption, exposing humans to light periods during atypical hours, promoting an irregular food intake pattern and modifying shift workers' social and family routines.
It is known that shift work displays a role in the increase of BMI; therefore we propose, according to the findings focused in several variables analysed in these studies, that age, time of exposure, the time in which someone works, strain of job and psychosocial factors may contribute to potentialise the increase in anthropometric parameters.
Shift work and circadian cycle disruption
The major function of the circadian system is the internal cycling of physiological and metabolic events. In fact, many physiological processes display day–night rhythms. Feeding behaviour and lipid and carbohydrate metabolism are subject to daily variation, showing a circadian pattern(Reference Sookoian, Gemma and Fernandez Gianotti17). The temporal organisation of the human body has to be understood to appreciate the impact of night and shift work on humans. The body has not only structure in space as expressed by its gross and microscopic anatomy, but it also has a structure in time consisting of rhythms of numerous frequencies superimposed on trends of development and ageing(Reference Haus and Smolensky5).
The temporal organisation of organic functions accounts for each activity being carried out during a particular hour of the day. This fact creates a series of controlled and rigorous procedures. The rhythmic variations encountered vary in period from milliseconds, as in individual nerve cells, to minutes or hours (ultradian rhythms) and to longer periods as in the menstrual cycle in women and seasonal periods (circannual rhythms) in both men and women. The rhythms most studied are related to 24 h, which determines the circadian rhythm expression proposed by Halberg(Reference Halberg18). Many of these rhythms are genetically fixed and the genes and their products have been characterised in different mammalian species and in humans. Like the sleep–wake cycle, feeding behaviour is under circadian control(Reference Follenius, Brandenberger and Hietter19–Reference Yang, Liu and Weidenhammer26). Some studies have shown the influence of circadian rhythm of feeding behaviour on body-weight regulation. For example, obese animal models show this disruption in feeding rhythm(Reference Yoshimatsu, Machidori and Doi27, Reference Hanada, Teranishi and Pearson28).
In all phyla, circadian rhythms have been demonstrated and species-specific dedicated clock genes have been found in model systems by reverse genetics. In humans, the mechanisms of the molecular clock remain hypothetical, as identification of human clock genes are predominantly based on their sequence similarity with those in other animals(Reference Allebrandt and Roenneberg29). In mammals, a clock centre (pacemaker) resides in the suprachiasmatic nucleus (SCN) located above the crossing of the optic nerves. The circadian clock controls physiology from gene expression to complex behaviours (for example, sleep and performance). This internal control is synchronised to the exogenous environment through signals, such as transient night and day cycles, where light is captured by retinal and transduced to the SCN via collaterals of the optic nerve where they synchronise the circa-daily rhythm produced by SCN neurons to exactly 24 h. Via its rhythmic outputs, the SCN coordinates all the cellular circadian clocks, including clocks from adipose tissue, to adapt physiology to the Earth's rotation(Reference Allebrandt and Roenneberg29).
Circadian molecular clocks driven by autoregulatory transcription–translation feedback loops consist of a pair of activator proteins, CLOCK and BMAL-1, in mammals that induce the transcription of a pair of repressor genes Per and Cry, additionally regulated by modifiers(Reference Lamont, Legault-Coutu and Cermakian30). These proteins have been found in the neural circadian master clock, SCN, and in several peripheral tissues including the adipose(Reference Kohsaka and Bass31). The peripheral clocks are synchronised through sympathetic outputs and the controlled secretion of circulating glucocorticoids, melatonin, and other mediators. These peripheral clock genes are similar to those present in the SCN neurons, although only the latter seem to be self-sustained. It is still unclear how these peripheral clocks are synchronised by the central SCN clock(Reference Kohsaka and Bass31, Reference Zvonic, Ptitsyn and Conrad32). Recent molecular studies revealed the direct coupling of clock genes and the regulation of metabolism(Reference Kohsaka and Bass31–Reference Yang, Downes and Yu33). Genetic mutations or deletions have implicated the peripheral clock genes in the regulation of glucose homeostasis(Reference Rudic, McNamara and Curtis34), lipid synthesis(Reference Turek, Joshu and Kohsaka35) and adipogenesis(Reference Shimba, Ishii and Ohta36), which are associated with obesity and type 2 diabetes mellitus. The role for Bmal1 and Clock in the regulation of glucose homeostasis was shown through the inactivation of the known clock components Bmal1 (Mop3) and Clock suppressing the diurnal variation in glucose and TAG. Gluconeogenesis is abolished by deletion of Bmal1 and is depressed in Clock mutants, but the feedback response of corticosterone and glucagon to insulin-induced hypoglycaemia is retained. In addition, a high-fat diet modulates carbohydrate metabolism by amplifying circadian variation in glucose tolerance and insulin sensitivity(Reference Rudic, McNamara and Curtis34). These genes are enrolled in the control of insulin-induced hypoglycaemia. Furthermore, when dietary cues are desynchronised, as happens in shift workers, the modification of metabolic homeostasis occurs via interactions of these genes with peripheral molecular clocks.
The mechanisms underlying internal desynchronisation have been mainly investigated in experimental animals with protocols that induce phase shifts of the light–dark cycle and thus modify the activity of the SCN. Salgado-Delgado et al. (Reference Salgado-Delgado, Angeles-Castellanos and Buijs37) developed an animal model of night work in which the light–dark cycle remained stable and where rats were required to be active in a rotating wheel for 8 h daily during their sleeping phase. This group was compared with rats that worked in the wheel during their activity phase and with undisturbed rats. They provided evidence that forced activity during the sleeping phase alters not only activity, but also the temporal pattern of food intake. As a consequence, these rats showed a loss of glucose rhythmicity and a reversed rhythm of TAG. In contrast, rats that worked during their activity phase did not show such changes and exhibited metabolic rhythms similar to those of the controls. The authors suggest that, in night workers, the combination of work and eating during working hours may be the cause of internal desynchronisation(Reference Salgado-Delgado, Angeles-Castellanos and Buijs37). This response takes place once the complete adaptation to the inversed phase is practically impossible. In shift work, central and peripheral oscillators must adapt to a new rhythmicity imposed by the work schedule. This modification requires time. Human rhythms are synchronised to diurnal activity by the environmental light–dark cycle and social routine, then undergo phase readjustment when forced to adhere to a new sleep–wake pattern. The central and peripheral oscillators will try to follow the new schedule, but this adaptation does not occur immediately. It is necessary for some sleep–wake cycles to adjust to the changed phase of the environmental synchroniser. It is known that even after a prolonged duration of time on shift, only a minority of night workers shows phase adaptation of their circadian system to the nocturnal activity pattern. The majority either shows no change in most of the variables examined or shows a rhythm disruption with some intermediate phase alterations(Reference Haus and Smolensky5, Reference Simon, Weibel and Brandenberger38).
The circadian rhythms of individuals synchronise the environment through the light–dark phase and social rhythm. In night–day alterations, such as in shift workers, alterations in social routine and/or meal times are described as desynchronisation. Different rhythms previously synchronised express themselves in different periods, where central and peripheral oscillators must adapt to a new rhythmicity. This modification demands some time. Rhythms of an individual, synchronised to diurnal activity by the environmental light–dark cycle and social routine, must undergo phase readjustment when forced to adhere to a new activity–sleep schedule due to shift work, for example. Symptoms comparable with jet lag, with gastrointestinal complaints, fatigue and sleepiness are often experienced by shift workers during the scheduled wake periods, and poor sleep during the daytime sleep attempts(Reference Knutsson39). The explanation of the phenotypic expression of obesity is a complex mechanism which involves mutations of clock genes, altered glucosic and lipidic metabolism, reduced thermogenic response due to a night eating pattern and disruption of neurohumoral factors, such as leptin and ghrelin and desynchronisation of clock genes presented in adipocyte cells. The disease model of shift work proposed in the present study is shown in Fig. 2.

Fig. 2 The disease model of shift work adapted from Knutsson(Reference Knutsson39).
Shift work and eating pattern
Some studies suggest indirectly that the metabolic efficiency of the diet is different depending on the time that food is eaten(Reference Simon, Weibel and Brandenberger38, Reference Weststrate, Weys and Poortvliet40–Reference Lennernas, Hambraeus and Akerstedt55). It was shown in the same subjects that there was a relative body-weight gain in humans when food was available only in the evening as compared with availability in the morning(Reference Romon, Edme and Boulenguez47).
Studies of the effects of shift work on eating habits and nutritient intake have previously been conducted(Reference Romon, Beuscart and Frimat46, Reference Pasqua and Moreno51, Reference Morikawa, Miura and Sasaki52, Reference Lennernas, Hambraeus and Akerstedt55–Reference de Assis, Kupek and Nahas57). Most studies did not find a difference between shift workers and daytime workers with respect to their total energy intake and their macronutrient intake. Instead, many reports found that there were changes in eating habits and food selection in shift workers. A cross-sectional study found that among subjects aged 30 years or more, the total energy intake was the highest among shift workers involved in midnight shifts. They did not find significant differences in nutrient intakes between day workers and shift workers without a midnight shift. It seems that the impact of shift work on nutrient intake differed by age and the type of shift work(Reference Morikawa, Miura and Sasaki52).
Workers who usually eat more energy in the evening seem to have a greater body weight. An experimental study was conducted to measure energy expenditure in nine non-obese young men selected randomly to receive a meal in one of three sessions (09.00, 17.00 or 01.00 hours). The snacks were the same for all participants. Energy expenditure was measured by indirect calorimetry 1 h before and for 6 h after the snack. The study showed a clear difference in the energy expenditure response to the same meal, depending on the circadian stage during which it was consumed. Morning diet-induced thermogenesis was significantly higher compared with afternoon and night. Therefore, the time at which a meal is consumed may affect the thermogenic response(Reference Romon, Edme and Boulenguez47). This fact may be related to shift workers' weight gain, since they seem to show a desynchronisation in lipid consumption during the day and a higher intake of this macronutrient through foods such as snacks. Associated with changes regarding eating pattern, sleep deprivation may contribute to the acceleration or triggering of metabolic disturbances, such as glucose intolerance, insulin resistance and dyslipidaemia(Reference Crispim, Zalcman and Dáttilo58).
Besides that, genes regulate body weight and food intake. The general model of intake regulation also involves environmental and psychological factors such as the social facilitation of eating, diurnal rhythms of intake, anxiety traits and restrained behaviour(Reference de Castro59). The role of genetics in shift work has not been clear until now; however, we can hypothesise that the phenomenon of desynchronisation may involve the genic expression of these genes, contributing to shift workers' increased body weight and waist circumference. This idea corroborates the findings concerning shift workers' eating patterns and suggests the delay of entrainment of rhythms coordinated by these genes. Further experimental research should investigate how these genes response to a desynchronised model.
Shift work v. metabolic disturbances
Shift work is accompanied by a greater incidence of many medical disorders such as cardiovascular, metabolic, gastrointestinal and sleep disorders(Reference Knutsson39, Reference De Bacquer, Van Risseghem and Clays60–Reference Conway, Campanini and Sartori65). Previous studies have demonstrated that risks increase according to exposure, such as hypertension, diabetes, coronary artery disease and weight gain(Reference Morikawa, Nakagawa and Miura8, Reference Morikawa, Nakagawa and Miura66, Reference Morikawa, Nakagawa and Miura67).
Apparently, the risk of shift work has been equated to the risk of smoking one pack of cigarettes per d. Coronary artery disease rates rise with exposure to shift work, even when controlled for other risk factors and confounding variables(Reference Whitehead, Thomas and Slapper68).
Some studies have demonstrated a relationship between shift work and metabolic alterations(Reference Weibel and Brandenberger41, Reference Ribeiro, Hampton and Morgan48, Reference Karlsson, Knutsson and Lindahl69–78). A study evaluating insulin resistance showed that shift workers who were aged 50 years old or younger showed insulin resistance more frequently than day workers(Reference Nagaya, Yoshida and Takahashi71). Another study, involving 300 workers from an Austrian refinery, demonstrated a prevalence of endocrine and metabolic diseases among shift workers of about 3·5 %: 1·5 % in day workers and 2·8 % in workers who alternated shifts(Reference Koller, Kundi and Cervinka77).
The Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) highlights the importance of treating patients with the metabolic syndrome to prevent CVD(78). The constellation of metabolic abnormalities, called the metabolic syndrome, includes glucose intolerance (impaired glucose tolerance, or impaired fasting glucose), insulin resistance, central obesity, dyslipidaemia and hypertension, predisposing subjects to an increased risk of type 2 diabetes and CVD(78). Because of variations in definitions and samples studied, the prevalence of the metabolic syndrome in some studies differed. Analysis of data from the Third National Health and Nutrition Examination Survey (1988–94), based on 8814 subjects aged 20 years or older, found, after adjustment for age, a metabolic syndrome prevalence of 23·7 %. This prevalence increased from 6·7 % among participants aged 20–29 years to 43·5 % for participants aged 60–69 years. It seems that sex did not make a difference to metabolic syndrome diagnosis: in men, 24·0 %; in women, 23·4 %. The 2000 census data showed that 47 million US residents have the metabolic syndrome(Reference Ford, Giles and Dietz79).
The most accepted and unifying hypothesis to describe the pathophysiology of the metabolic syndrome is insulin resistance. Insulin resistance has traditionally been defined from a glucocentric view, i.e. a defect in insulin action results in fasting hyperinsulinaemia to maintain euglycaemia. Yet, even before fasting hyperinsulinaemia develops, postprandial hyperinsulinaemia exists. A major contributor to the development of insulin resistance is an overabundance of circulating fatty acids. Fatty acids are also derived through the lipolysis of TAG-rich lipoproteins in tissues by the action of lipoprotein lipase. Insulin is important to both antilipolysis and the stimulation of lipoprotein lipase. Of note, the most sensitive pathway of insulin action is the inhibition of lipolysis in adipose tissue. Thus, when insulin resistance develops; the increased amount of lipolysis of stored TAG molecules in adipose tissue produces more fatty acids, which could further inhibit the antilipolytic effect of insulin, creating additional lipolysis. Upon reaching insulin-sensitive tissues, excessive fatty acids create insulin resistance by the added substrate availability and by modifying downstream signalling(Reference Eckel, Grundy and Zimmet80).
Previous studies have shown an association between shift work and lipid profile disturbances, where these disturbances could be due to internal desynchronisation(Reference Romon, Le Fur and Lebel45, Reference Lennernas, Akerstedt and Hambraeus54, Reference Ghiasvand, Heshmat and Golpira76). Subjects who worked three shifts showed higher total cholesterol and TAG levels than did day workers and full-time workers. The authors reported that 69 % of three-shift workers did not engage in any physical recreational activity. Three-shift work was independently associated with total cholesterol(Reference Ghiasvand, Heshmat and Golpira76). Perhaps stress, a common condition in these individuals, could induce hypercholesterolaemia. Another possibility is that shift workers' food intake is more abundant in cholesterol, since it is known that their lipid consumption is higher than regular workers(Reference de Assis, Nahas and Bellisle56, Reference de Assis, Kupek and Nahas57). Research has shown that high serum total cholesterol and LDL-cholesterol levels were more common in shift workers than in day workers. This finding persisted after adjustment was made for age and food type. However, in disagreement with other authors, this study did not show difference in the prevalence of HDL-cholesterol, TAG and fasting blood glucose and hypertension between shift working and day working(Reference Ghiasvand, Heshmat and Golpira76).
The fact that shift workers present a chronically reversed sleep–wake cycle may be associated with the higher incidence of the metabolic syndrome in this population(Reference Biggi, Consonni and Galluzzo70, Reference Holmback, Forslund and Lowden81). The imposed desynchronisation may be responsible for changes in the metabolism and secretion patterns of endocrine factors. This circadian rhythm in neurological, endocrine, thermoregulatory and other body functions may resynchronise only slowly after the abrupt phase shift common to rotating shift work.
Shift work is related to circadian rhythmicity disruption, which occurs with alterations in one or more of the pathological components of the metabolic syndrome(Reference Karlsson, Knutsson and Lindahl10, Reference Tanofsky-Kraff and Yanovski82). Deletion of the Clock and Bmal1 genes results not only in circadian disruption, but also in metabolic abnormalities of lipid and glucose homeostasis – a phenotype similar to the metabolic syndrome(Reference Rudic, McNamara and Curtis34, Reference Turek, Joshu and Kohsaka35) – which suggests that this clock disruption directly influences the metabolism. This is supported by a study that reported the development of the metabolic syndrome in Clock mutant mice(Reference Turek, Joshu and Kohsaka35), showing that a loss of function in this gene results in altered patterns of food intake. Then, these animals present an increase in their food consumption, becoming obese and developing hyperglycaemia and dyslipidaemia. The parallel also occurs in the development of adipocyte hypertrophy. It seems that mutations in Bmal1 and Clock not only modify the diurnal variation in levels of plasma glucose and TAG, but also influence the progress of glucose impairment and insulin resistance, once submitted to a high-fat diet. Apparently the total energy intake of shift workers is not the problem, as their consumption is very similar to that of day workers. We hypothesise that the composition of food associated with night eating may play a role more relevant than the energy. This statement is evidenced by studies regarding food intake. Individuals engaged in this kind of labour pattern do not eat more energy than day workers, but they present an elevated consumption of snacks, which are high-fat meals. This kind of food behaviour becomes even more noxious considering the time that meals are taken, which explains such alterations presented in shift workers.
Physical inactivity and overweight/obesity contribute approximately 50 % to the insulin resistance process and glucose intolerance in healthy, normotensive and non-diabetic subjects(Reference Wolk and Somers73). Other factors that may contribute to these disturbances are being studied, such as alterations in cyclic endocrine rhythms involved in shift work. In healthy subjects, glucose tolerance decreases during the day. Studies have shown that both glucose and insulin responses seem to be mediated by circadian rhythms(Reference Fukushima, Lupien and Bray21).
Studies have found that the insulin response to glucose may result in β-cell circadian alterations, producing a responsive that is greater in the morning and decreases during the day(Reference Smith43). Shift work as well as shift workers' behaviour may lead to the disruption of the endocrine circadian rhythm, such as levels of glucose(Reference Morgan, Arendt and Owens75, Reference Copertaro, Bracci and Barbaresi83, Reference Muller, Keck and Zimmermann84), insulin(Reference Qin, Li and Wang49, Reference Nagaya, Yoshida and Takahashi71, Reference Morgan, Arendt and Owens75), leptin(Reference Asakuma, Hiraku and Kurose85–Reference Motivala, Tomiyama and Ziegler89), TAG(Reference Karlsson, Knutsson and Lindahl69, Reference Biggi, Consonni and Galluzzo70, Reference Ghiasvand, Heshmat and Golpira76, Reference Copertaro, Bracci and Barbaresi83), and total cholesterol(Reference Nakamura, Shimai and Kikuchi13, Reference Lennernas, Akerstedt and Hambraeus54, Reference Biggi, Consonni and Galluzzo70, Reference Ghiasvand, Heshmat and Golpira76) and its fractions(Reference Lennernas, Akerstedt and Hambraeus54, Reference Copertaro, Bracci and Barbaresi83). Besides, Copertaro et al. found a higher prevalence of the metabolic syndrome among shift workers than daytime workers, when using the International Diabetes Federation diagnostic criteria. In accordance with these data, shift workers were also more likely than regular workers to have a high waist circumference(Reference Copertaro, Bracci and Barbaresi83).
Despite the fact that Di Lorenzo et al. (Reference Di Lorenzo, De Pergola and Zocchetti2) found a relationship between shift work and BMI, they were unable to reproduce this finding with regard to total cholesterol serum levels, TAG and HDL-cholesterol. These results may be attributed to differences in dietary habits and also to genetic differences related to geographic region. On the other hand, both TAG and HDL-cholesterol were independently associated with serum insulin concentration. These results permitted a connection between insulin resistance and higher TAG and lower HDL-cholesterol, indicating the metabolic syndrome. Insulin levels were significantly associated with BMI, but there was no relation to shift work. The authors hypothesised that shift work could have a role in BMI and body fat increase, producing insulin resistance and altering glucose metabolism. Systolic blood pressure and glucose tolerance differed significantly in shift workers(Reference Di Lorenzo, De Pergola and Zocchetti2). Unfortunately, systolic blood pressure was not analysed in a chronobiological scope. Therefore, its increase in shift workers could be explained by either rhythm desynchronisation or overweight or an interaction between the two variables. In conclusion, even in the Mediterranean region, shift work exerts an influence on obesity, CVD and the metabolic syndrome.
Subjects who worked three shifts showed higher total cholesterol and TAG levels than did day workers and full-time workers. The authors reported that 69 % of three-shift workers did not engage in any physical recreational activity. Three-shift work was independently associated with total cholesterol(Reference Ghiasvand, Heshmat and Golpira76). Perhaps stress, a common condition in these individuals, could induce hypercholesterolaemia. Another possibility is that shift workers' food intake is more abundant in cholesterol, since it is known that their lipid consumption is higher than regular workers(Reference de Assis, Nahas and Bellisle56, Reference de Assis, Kupek and Nahas57). Research has shown that high serum total cholesterol and LDL-cholesterol levels were more common in shift workers than in day workers. This finding persisted after adjustment was made for age and food type. However, there was no difference in the prevalence of HDL-cholesterol, TAG and fasting blood glucose and hypertension between shift working and day working. The authors concluded that shift work is a risk factor for lipid profile disturbances(Reference Ghiasvand, Heshmat and Golpira76).
In the meantime, shift workers present higher BMI and waist circumference than day workers. These results may suggest a role played by shift work on the development and/or the early clinic manifestations of metabolic disturbances, becoming a risk factor for the metabolic syndrome.
In chronobiological approach it is important to highlight that the hormones leptin and ghrelin, anorexin and orexin are secreted, as are the majority of hormones, in a circadian pattern(Reference Stutz, Staszkiewicz and Ptitsyn25, Reference Yang, Liu and Weidenhammer26, Reference Zvonic, Ptitsyn and Conrad32, Reference Yang, Downes and Yu33, Reference Shea, Hilton and Orlova72, Reference Baskin, Figlewicz Lattemann and Seeley86, Reference Motivala, Tomiyama and Ziegler89–Reference Natalucci, Riedl and Gleiss94). Then we may hypothesise that light exposure at night could contribute to a decreased and/or delay in the secretion of leptin, which is secreted usually at night with its acrophasis at around midnight. This could contribute to an enhanced hunger and food intake pattern associated with an increase in ghrelin levels, leading to weight gain and visceral fat accumulation in the abdominal region. Simultaneously with stress, job strain and psychosocial factors, shift workers are then predisposed to cortisol hypersecretion, with hyperstimulation from the hypothalamic–pituitary–adrenal axis leading adipose tissue to produce even more fat tissue. Simultaneously, metabolic disturbances could be due to desynchronised rhythm or shift workers' lifestyle, or both. In fact, stress and lifestyle are potent mediators in the development of metabolic conditions in shift workers. The existence of an active circadian clock in adipocytes suggests that there is a temporal component to the regulation of adipose tissue function. Recent evidence connecting circadian dysfunction to obesity and the metabolic syndrome strongly supports this notion(Reference Rudic, McNamara and Curtis34, Reference Turek, Joshu and Kohsaka35). Metabolism and maintenance of energy homeostasis require functional coordination among individual adipose depots and other metabolically active tissue sites, to ensure proper nutrient/energy flux and substrate use by the organism. Desynchronisation produced by feeding or alternative entrainment mechanisms may lead to defective substrate use, resulting in the disruption of metabolic pathways leading to intramyocellular lipid accumulation, and insulin resistance.
Experimental studies may be very helpful to understand these mechanisms. For example, inverting the sleep–wake cycle and food intake pattern of animals may provide evidence that hormones concerning body weight, adipose tissue and food intake suffer the circadian misalignment provoked by the alteration of the light–dark cycle. Further research similar to that proposed above may be able to investigate new conceptions and possible mechanisms supporting the chronobiological argument of a disruption on the pacemakers, including the oscillators present in adipocytes.
Discussion
Shift work has long been unrecognised as an occupational health hazard up until now. Currently research in the field has shown limited evidence available, due to underpowered studies, since the most common design was cross-sectional. Articles analysed did not describe protocols to control anthropometric measures, which could cause bias and impairment in the interpretation of data, since variables such as weight and height were self-reported. Studies involving body weight must show a cohesive and accurate design, mostly because a series of variables, such as lifestyle and eating habits, may be strong confounding factors. Also, the inclusion of subjects with different lifestyles accounts for biases, even though they may be from the same work place. Concerning sample selection, authors must minimise potential confounders. It is important to maintain homogeneity between shift group and control group for years on shift, age and a detailed anamnesis about previous body weight and clinical and surgical treatments for weight control. However, we were able to demonstrate associations between shift work and increases in BMI and metabolic disturbances, supported by experimental studies that had a more rigorous design.
Obesity now represents the most prevalent nutritional problem, with a prevalence of 300 million adults worldwide, given the limited availability of effective treatment of weight problems. It is known that this pathology consists of a strong risk factor for lipidic profile disturbances, central obesity, insulin resistance, circulatory disease and the metabolic syndrome. At the core of the association between the sleep–wake cycle and obesity may be a molecular mechanism intrinsic to all eukaryotic cells and organisms, namely circadian oscillators. Recent investigations suggest that the causes of obesity involve a complex interplay of genetic, environmental, psychobehavioural, endocrine, metabolic, cultural and socio-economic factors. Recently, circadian oscillator genes in adipose tissue have brought significant metabolic implications; their characterisation may provide potential therapeutic relevance. Current treatments for obesity have been largely unsuccessful in maintaining long-term weight loss, demonstrating the urgent need for new insight into mechanisms that may lead to obesity and altered metabolism. From now on, it is important to provide guidelines concerning a better adaptation and to monitor shift work to reduce its risks. The field of occupational medicine must design a specific protocol composed of clinical and laboratorial features presented in shift workers. The clinical examination should be done with a more frequent periodicity. For example, it would be helpful if shift workers were submitted to an evaluation directed to screening the metabolic syndrome, diabetes mellitus and CVD. A biochemical protocol involving total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG and glucose serum may be supportive to an early diagnosis or to identify subjects at an increased risk of the development of these pathologies. Mainly, it is essential to take a very careful look at minimising alterations in these biochemical features. Anthropometric measures, such as body weight, BMI and waist circumference, should also be taken in a clinical examination. In order to minimise damage, companies should provide dietary and lifestyle counselling, promoting healthier habits. Those companies that provide snacks and/or meals during shifts should make a dietary plan specifically for shift workers, once their risks concerning metabolic disturbances are known. An exercise programme could promote a better lifestyle, becoming a protection factor to the maintenance of shift workers' health. A sleep education programme could also be interesting to encourage sleep hygiene practices. With regard to shift schedule, intervention studies indicated that the introduction of a better schedule improves biomarkers related to the metabolic syndrome, while a worse shift schedule promotes weight gain in this population(Reference Boggild and Jeppesen95).
The role of the circadian clock mechanism in metabolic conditions in shift workers represents an exciting new field of study in pursuit of the causes of the increasing prevalence of obesity. The elucidation of the link between the circadian clock disruption and metabolic disturbances in shift workers may have profound implications on the timing of obesity therapies. Future studies are needed to elucidate circadian alterations in shift workers, although it is also essential to have a rigorous design concerning sample homogeneity and the variables examined.
Table 1 summarises articles covering topics featured in the present review.
Table 1 Overview of studies on the effects of shift work on BMI, circadian disruption and metabolic disturbances
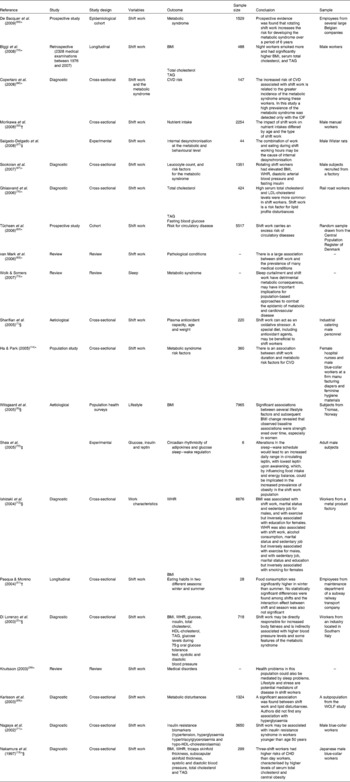
IDF, International Diabetes Federation; WHR, waist:hip ratio.
* Shift work and BMI.
† Shift work and circadian cycle disruption.
‡ Shift work and eating patterns.
§ Shift work and the metabolic syndrome.
Acknowledgements
The present review was supported by the Graduate Research Group (GPPG) at Hospital de Clínicas de Porto Alegre, Brazil.
The authors thank the Post-Graduation Program in Medical Sciences, School of Medicine, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil and Fundo de Incentivo à Pesquisa e Eventos (FIPE) at Hospital de Clínicas de Porto Alegre, Brazil, for supporting the present review.
L. C. A. designed the study, wrote the protocol, reviewed studies, drafted the article and interpreted the data. R. L. and G. D. were responsible for drafting the article. W. C. and M. P. H. participated in study design, drafting the article and final approval of this version.
There was no financial relationship between any of the authors or any commercial interest in the outcome of the present study.





