Introduction
Carotenoids are typically C-40 based tetraterpenoid secondary plant compounds, although more recently, also C-30(Reference Umeno, Tobias and Arnold1) and C-50(Reference Giuffrida, Sutthiwong and Dugo2) based carotenoids in bacteria have been described, resulting in over 1100 known carotenoids(Reference Yabuzaki3). Carotenoids do occur in plants, bacteria, fungi and animals, though the latter are generally unable to produce them, most likely due to evolutionary loss of functional genes encoding the necessary enzymes for biosynthesis. Many carotenoids are pigments of yellow or orange colour and are associated in plants with chlorophyll, improving the photosynthesis process by enhancing light harvesting in the blue spectrum and protecting from photo-oxidative damage(Reference Hashimoto, Uragami and Cogdell4).
Carotenoids have been met with much interest, due to the association of their dietary intake as well as circulating blood concentrations with reduced incidence of chronic diseases. For example, subjects with the highest concentrations of circulating β-carotene showed decreased all-cause mortality compared with those with lowest concentrations(Reference Buijsse, Feskens and Schlettwein-Gsell5). In a recent meta-analysis, both dietary intake of various carotenoids (β-carotene, α-carotene and β-cryptoxanthin) and circulating concentrations in the blood were related to decreased total mortality(Reference Aune, Keum and Giovannucci6). Based on these assumed health benefits, a health index has been proposed, with plasma/serum concentrations below 1 µM of total carotenoids being related to a significantly increased risk for chronic diseases(Reference Donaldson7). These mainly correlation-based studies indicate beneficial effects for high physiological levels of carotenoids and, in consequence, support a recommendation for a high intake of fruits and vegetables rich in carotenoids (reviewed in ref.(Reference Böhm, Lietz and Olmedilla-Alonso8)). Besides carotenoids, a high intake of fruits and vegetables is also associated with a healthier lifestyle, including regular exercise, less smoking and less alcohol abuse(Reference Nebeling, Forman and Graubard9), as well as generally healthier food intake, including a lower intake of processed meat(Reference Segovia-Siapco, Burkholder-Cooley and Haddad Tabrizi10). Furthermore, lower endogenous carotenoid levels were also described to correlate with an increased chronic inflammatory status and may simply function as a health status biomarker, not only due to a lower intake of fruits and vegetables but also because of a higher degradation of carotenoids due to a chronic pro-inflammatory environment(Reference Calder, Ahluwalia and Brouns11,Reference Kritchevsky, Bush and Pahor12) . Consequently, higher physiological carotenoid levels in humans could simply function as biomarker of a healthier lifestyle, a higher intake of fruits and vegetables and a better health status(Reference Nebeling, Forman and Graubard9), as indicated in Fig. 1. Despite these positive associations, also negative effects have been reported, i.e. increased risk for lung cancer in smokers upon administering high doses of supplemental β-carotene (20–30 mg/d) for several years(Reference Omenn, Goodman and Thornquist13,Reference Blumberg14) .
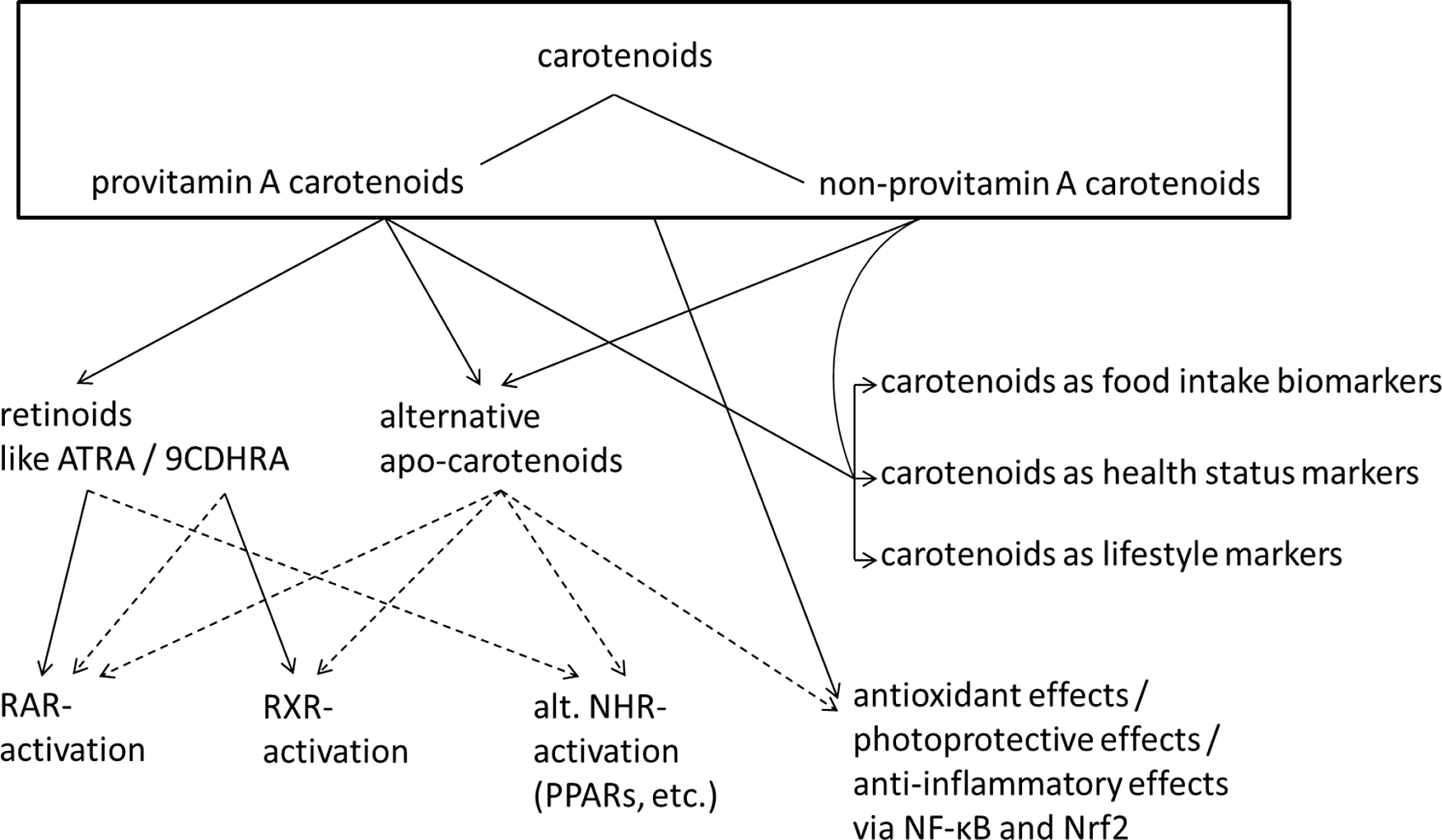
Fig. 1. Summary of carotenoid mediated health effects. Suggested and not conclusively proven connections are indicated with dashed lines. Abbreviations: ATRA, all-trans retinoic acid; 9CDHRA, 9-cis-13,14-dihydroretinoic acid; RAR, retinoic acid receptor; RXR, retinoid X receptor; NHR, nuclear hormone receptor; alt., alternative; PPARs, peroxisome proliferator-activated receptor.
Carotenoids have been associated with the prevention and amelioration of chronic diseases in several ways. In addition to acting as mere indicators of a diet rich in plant-based foods(Reference Burrows, Williams and Rollo15) and, thus, healthy eating patterns, several carotenoids, i.e. those with provitamin A potential, can act as essential micronutrients. These include α-carotene, β-carotene and β-cryptoxanthin, which can be converted by β-carotene oxygenase 1 (BCO1) in the human body into vitamin A active compounds, important for subjects with low intake of preformed vitamin A, i.e. mainly retinyl esters, such as vegetarians or in more rural non-Westernised societies with low meat or animal-based food intake(Reference Grune, Lietz and Palou16). Thus, regular provitamin A carotenoid intake by such people contributes to the prevention of vitamin A deficiency, which is indicated by serum levels of retinol less then 0.7 µM(Reference Foster17). Night blindness and xerophthalmia are vitamin A deficiency syndromes(Reference West18), though mainly of relevance in developing countries with co-occurring additional nutrient deficiencies(Reference Vijayaraghavan19,Reference Chiu, Dillon and Watson20) , while abnormalities such as a reduced immunological competence and pulmonological disturbances are more frequent vitamin A deficiency disorders in humans with main relevance for children in Western society(Reference Timoneda, Rodriguez-Fernandez and Zaragoza21,Reference Wirth, Petry and Tanumihardjo22) .
Rather specifically, the carotenoids lutein and zeaxanthin, together with the in vivo formed meso-zeaxanthin(Reference Nolan, Meagher, Kashani and Beatty23), are important in maintaining the integrity of the macula in the retina of the human eye, via photo-protection from blue light and appear to improve visual aspects in subjects with age-related macular degeneration(Reference Arunkumar, Calvo and Conrady24), the most common cause of vision loss in the elderly.
In general, carotenoids can act, at least in vitro, as antioxidants (Fig. 1), acting as scavengers of singlet oxygen or reactive lipid peroxides(Reference Krinsky and Johnson25), protecting cell membranes. To which extent this function contributes to the observed health effects with human relevance is unclear, and their antioxidant properties in vivo have to some extent been questioned(Reference Erdman, Ford and Lindshield26,Reference van Helden, Keijer and Heil27) . Finally, carotenoids and their metabolites, the apo-carotenoids, are likely to interact with many cellular targets such as transcription factors and nuclear hormone receptors. Important interactions could include the interruption of the NF-κB pathway, reducing the activation of further downstream pro-inflammatory genes, resulting in the sequestration of several cytokines (TNFα, IL6), nitric oxide and cyclooxygenase 2, among other(Reference Kaulmann and Bohn28). Similarly, binding of carotenoids to the bound NRF2 transcription factor could result in its release and nuclear translocation, fostering the body’s own antioxidant defence system, including increased expression of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidases (GPX)(Reference Kaulmann and Bohn28). Both increased inflammation and oxidative stress are related to several cardiometabolic complications, including type 2 diabetes(Reference Zheng, Guo and Jia29) and metabolic syndrome(Reference Grandl and Wolfrum30). Through their involvement as potential antioxidants and their interaction with many cellular targets, carotenoids have also been proposed to reduce the risk of several types of cancer. For instance, in a recent meta-analysis, subjects with higher carotenoid intake were shown to have lower breast cancer risk(Reference Hu, Wang Yi and Zhang31), although this may have been related to confounding factors such as increased intake of fibre. Some randomised controlled studies giving additional antioxidants such as selenium and α-tocopherol(Reference Hu, Wang Yi and Zhang31) and zinc and selenium together with β-carotene(Reference Hercberg, Galan and Preziosi32) have also suggested positive effects on health, especially regarding cerebrovascular disease mortality and all-cause mortality in men, respectively.
Well known is the interplay of carotenoid metabolites with nuclear hormone receptors (summarised in Fig. 1), mainly the retinoic acid receptors (RARs) and retinoid X receptors (RXRs) and further signalling with RXR liganded heterodimers, where peroxisome proliferator-activated receptors (PPARs) appear to play an important role(Reference Bohn, Desmarchelier and Sedef33–Reference Dulińska-Litewka, Sharoni and Hałubiec36) RARs and RXRs are involved in regulating the expression of a large number of genes involved in the cellular development and the immune system(Reference Savory, Edey and Hess37), and PPARs are involved in the metabolism and differentiation of adipocytes(Reference Bonet, Canas and Ribot38). Thus, carotenoids could contribute to improved immune function, including reduced risk of respiratory complications such as asthma, as reviewed previously(Reference Melo van Lent, Leermakers and Darweesh39) and also the reduction of perturbed lipid metabolism, a hallmark of metabolic syndrome(Reference Iqbal, Al Qarni and Hawwari40).
This review highlights the different mechanisms via which carotenoids may be related to various diseases, i.e. on the pathways involved in the respective diseases and how these are influenced by carotenoids, and emphasises common or diverging properties of these phytochemicals important for their potential health beneficial aspects.
Mechanisms of action transmitted by carotenoids
Antioxidant activities
Carotenoids as light-harvesting pigments have an important role in protecting the photosynthetic apparatus from reactive oxygen species (ROS), such as singlet oxygen, in plants and photosynthetic bacteria(Reference Frank and Brudvig41). They can scavenge singlet oxygen in simple solutions; however, their interactions in vivo are complex; aggregated carotenoids and those spanning cell membranes can reduce their quenching efficiency. Carotenoids can also quench other free radicals to form carotene radicals with different properties(Reference Böhm, Edge and Truscott42). These antioxidant effects include preventing oxidation of LDL particles(Reference Kiokias, Proestos and Oreopoulou43), and likely inhibiting oxidation of lipids in brain cells(Reference Craft, Haitema and Garnett44), prevention of oxidation of polyunsaturated lipids in the retina(Reference Saccà, Cutolo and Ferrari45), and also by limiting oxidation of lipids during digestion in the gastro-intestinal lumen(Reference Kanner and Lapidot46). Therefore, in complex environments, the antioxidant effects of carotenoids depend on the combined properties of the radicals involved, including their concentrations.
Additionally, some carotenoids or their radicals can modulate cell signalling to induce antioxidant and detoxification enzymes, cell proliferation and apoptosis(Reference Kanner and Lapidot46,Reference Palozza, Catalano and Simone47) . These complex interactions are reflected in the discrepancies between the epidemiological studies, suggesting beneficial effects of carotenoids within the normal dietary range, while it has been found difficult to prove these effects by large interventional clinical trials employing in part supra-physiological doses. Nevertheless, carotenoids are also being extensively used as food colourants(Reference De Mejia, Zhang and Penta48), dietary supplements, components of sunscreens and other cosmetic products. Their use and function will be described in the context of anti-cancer and skin-protection mechanisms.
Lycopene is among the most effective carotenoid free-radical scavengers, because the free radical compound formed with lycopene is more stable than that formed with other compounds in this group, which indicates that it can reduce, through electron transfer, also other carotenoids(Reference Krinsky and Johnson25). In vitro studies conducted by Wawrzyniak(Reference Wawrzyniak49) found lycopene to be stable in acid and neutral gastric content, which was confirmed by the work of other authors(Reference Bilton, Gerber and Grolier50) and also in the upper intestine(Reference Kopec, Caris-Veyrat and Nowicki51). The addition of lycopene and sodium nitrite to stomach content at the same time caused a significant decrease in the content of lycopene, and significantly lowered the concentration of sodium nitrite(Reference Wawrzyniak49); lycopene decrease depended on the amount of nitrite added. In in vivo studies, a decrease in blood lycopene concentrations was observed in subjects consuming higher amounts of nitrite with food products(Reference Kalaycloǧlu and Erim52). In addition, oral administration of lycopene to animals showed a protective effect by lowering poisoning rates, i.e. the concentration of methemoglobin in whole blood of animals, and the concentration of nitrite and nitrate ions in the serum of rats poisoned with sodium nitrite(Reference Wawrzyniak49).
In an experiment carried out by Atanasowa and Pevitcharova(Reference Atanassova and Pevitcharova53), a beneficial effect of a diet with a lycopene content of 285–640 mg (per kg of diet) was shown to reduce animal mortality after an administration of a high dose (LD50) of sodium nitrite. This study implies that lycopene may be a compound with protective effects; although a positive contribution of e.g. vitamin C, β-carotene, polyphenols and dietary fiber is also possible, as tomato paste was the source of lycopene in the diet of animals. Tomato paste also decreased the risk of nitrosamine formation, after administration of sodium nitrite and aminopyrine – a nitrosamine precursor(Reference Atanasova-Goranova, Dimova and Pevicharova54). In contrast to the sodium-nitrate-only exposed group, rats also receiving 236 mg lycopene per kg of diet did not show circulating markers of liver damage, i.e. the activity of alanine and aspartate aminotransferase in blood were similar compared with the controls, and the livers retained their natural appearance compared with the poisoned group not receiving lycopene, where dystrophic changes occurred.
In conclusion, in healthy people, consuming cured meat (the main source of nitrite in the diet) together with vegetables and fruits is recommended, the latter as sources of carotenoids that may protect against the harmful, oxidative effects of sodium nitrite and the formation of methemoglobin. The International Agency for Research on Cancer (IARC) recognised this relationship between excessive consumption of processed meat (cured, treated at high temperatures or simmered) and the occurrence of large intestine, pancreas and prostate tumors in 2015(Reference Goodson, Lowe and Carpenter55).
Activation of antioxidant/anti-inflammatory cascades involving the transcription factors NF-κB and NRF2
The transcription factor NF-κB was discovered 30 years ago(Reference Sen and Baltimore56). It is well established that it plays a key role in adapting biological processes to environmental changes (for review, see refs.(Reference Hayden and Ghosh57–Reference Zhang, Lenardo and Baltimore59)). This is in particular due to a major effect on the immune system, modulating the expression of cytokines, growth factors or micro-RNAs. NF-κB is also involved in other gene regulation, which may have great repercussions on physiology and physiopathology processes, such as embryonic development, on the skin, the bones or the central nervous system. It also plays a key role in metabolic inflammation, proliferative and apoptotic responses, as well as in tumor formation. This great variety of effects is related to an activation mechanism associated with most stimuli.
The NF-κB family has five members in vertebrates: RELA (better known as P65), RELB, REL, NFKB1 (P50/P105) and NFKB2 (P52/P100). In non-stimulated cells, these proteins are assembled in homo- or heterodimers and kept inactive in the cytoplasm by their association with IκB proteins. The binding to IκB actually prohibits translocation of NF-κB to the nucleus. Under a great variety of stimuli, such as pro-inflammatory cytokines (TNFα, IL1β), bacterial lipopolysaccharides (LPS), phorbol esters, genotoxic agents, radiation, etc., IκB proteins are quickly phosphorylated by the protein IKKκ (IκB kinase κ), ubiquitinylated and finally cleaved by the proteasome. This activation pathway applies mainly to P65/P50 dimers. In addition to this conventional pathway, there is also an alternative activation pathway, in which the protein IKKκ, via the phosphorylation of P52, leads to the release of the complex P52/RELB. The NF-κB dimers released in this way then enter the nucleus and activate the transcription of specific target genes after binding to a κB sequence present in their promoting region. Among these target genes, we can mention anti-apoptotic genes (BCL2L1, BIRC1/2), inflammation-related genes (IL1, IL2, IL6, TNFα), genes involved in cell adhesion, growth factors, chemokines, micro-RNAs, etc.
The activation of NF-κB (P65/P50) depends both on the phosphorylation level of IκBκ as well as the bond affinity of P65 to its response element, which may be modified by the redox state of the cell. In fact, the P65 DNA binding domain contains a high number of reactive cysteines(Reference Liu, Colavitti and Rovira60), which is why NF-κB is considered to be a redox-sensitive transcription factor. Several carotenoids or derivatives influence the NF-κB signalling pathway(Reference Sharoni, Linnewiel-Hermoni and Khanin61,Reference Linnewiel-Hermoni, Motro and Miller62) . Therefore, they can act as anti-inflammatory compounds, in a large range of cell types where they modulate the expression of inflammation markers, via the modulation of P65 transcriptional activity. β-Carotene inhibits NF-κB signalling in human breast cancer cells(Reference Sowmya Shree, Yogendra Prasad and Arpitha63), in human oesophageal squamous cell carcinoma(Reference Zhu, Zhang and Li64) and in gastric epithelial AGS cells(Reference Kim, Seo and Kim65). These effects are related to an inhibition of P65 translocation in conjunction with inhibition of phosphorylation and breakdown of IκBκ.
Similar effects have been described for lycopene, on human hepatoma cells, where this compound reduces the binding capacities of P65 on its response elements(Reference Huang, Fan and Lin66). This can partly explain the effect of lycopene on MMP9 expression, the reduction of which is associated with a reduction in the processes involved in the migration of cancer metastases. Similar effects of lycopene on NF-κB activation have also been obtained in dendritic cells(Reference Kim, Kim and Ahn67) and prostate and breast cancer cells(Reference Assar, Vidalle and Chopra68). Interestingly, an impact of β-carotene oxygenase 2 (BCO2) on NF-κB activity and NF-κB translocation and DNA binding has been reported in prostate cancer, suggesting that BCO2 exerts a direct effect on NF-κB signalling, independently of its enzymatic role in lycopene metabolism(Reference Gong, Marisiddaiah and Zaripheh69).
The same applies to other carotenoids, such as astaxanthin, which thereby exhibits anti-inflammatory properties via inhibition of NF-kB signalling in non-small cell lung(Reference Cheng and Eroglu70), in human hepatocellular carcinoma(Reference Li, Dai and Xia71) and also shown in a hamster model of oral cancer(Reference Kavitha, Kowshik and Kishore72), and has been shown to display anti-inflammatory properties. Lutein acts on NF-κB signalling to block the P65 DNA binding domain, in proliferating breast cancer cells(Reference Chang, Zhang and Li73) and in gastric epithelial AGS cells(Reference Kim, Seo and Kim65). Fucoxanthin reduces NF-κB activity in human cervical cancer cells, leading to apoptosis(Reference Jin, Qiu and Shao74). Similar results were depicted in breast cancer cells (MCF-7 and MDA-MB-231;(Reference Rwigemera, Mamelona and Martin75)) and in human hepatoma cells(Reference Liu, Lim and Hu76).
In adipocytes, a strong impact of lycopene on NF-κB signalling was demonstrated. Indeed, lycopene and a chemically synthesised potential lycopene metabolite, apo-10′-lycopenoic acid, following incubation of 3T3-L1 adipocytes and adipose tissue explants, strongly reduced pro-inflammatory cytokines and chemokines via modulation of IKK protein phosphorylation(Reference Gouranton, Thabuis and Riolle77–Reference Marcotorchino, Romier and Gouranton79). Recently, similar effects of the two main lycopene isomers were observed, i.e. all-trans- and 5-cis lycopene(Reference Fenni, Astier and Bonnet80). Finally, these effects have been confirmed in vivo, in diet-induced obesity in mice supplemented with lycopene, where pro-inflammatory cytokines and chemokine expression and secretion were reduced as well as the p65 phosphorylation level(Reference Fenni, Hammou and Astier81).
NRF2 (NFE2L2) is another transcription factor that activates the antioxidant response element (ARE, also known as EpRE, electrophile response element) transcription system. ARE regulates the expression of antioxidant enzymes, such as CAT, SOD, GPXs and thioredoxin, and detoxifying enzymes such as heme oxygenase 1 (HO1), NAD(P)H dehydrogenase and quinone 1 (NQO1). These proteins have an important role in reducing the intracellular concentration of carcinogens and ROS and thus may reduce the progression of degenerative diseases such as cancer, osteoporosis and skin ageing, among others. Carotenoids such as lycopene, phytoene, phytofluene and astaxanthin that have been associated with a lower rate of progression of degenerative diseases were found to activate ARE in cancer cells at concentrations of 1–10 µM(Reference Ben-Dor, Steiner and Gheber82), in addition to several other phytochemicals, such as polyphenols, isothiocyanates and curcuminoids(Reference Yu and Kong83). In recent years, the list of carotenoids that activate ARE was extended, and this activity was found to be involved in the effect of carotenoids in various tissues and cellular systems in addition to cancer cells. For example, fucoxanthin, a marine carotenoid, increased NRF2 activation and HO1 expression in activated microglia cells(Reference Zhao, Kwon and Chun84). Similarly, fucoxanthin reduced UVB-induced erythema through up-regulation of the HO1 protein via the NRF2 pathway(Reference Rodríguez-Luna, Avila-Roman and Gonzalez-Rodgriguez85). The protective role of lutein against injury in rat skeletal muscle(Reference Cheng, Zhang and Yan86) and against β-amyloid-induced oxidative stress in cerebrovascular endothelial cells(Reference Liu, Liu and Zhao87) was associated with an up-regulation of NRF2. Astaxanthin had a similar effect in human umbilical vein endothelial cells(Reference Niu, Xuan and Jiang88). The role of NRF2 in mediating the protective effects of lycopene toward cancer was evident also in in vivo models. In a rat model of carcinogen-induced hepato-carcinogenesis, lycopene increased the expression of NRF2 and reversed the reduction in hepatic antioxidant enzymes (CAT, SOD, GPX) caused by the carcinogen(Reference Sahin, Tuzcu and Sahin89). Conversely, some studies have reported a down-regulation of the NRF2 protein(Reference Sowmya Shree, Yogendra Prasad and Arpitha63) or mRNA(Reference Talvas, Caris-Veyrat and Guy90) levels by carotenoids.
Under resting conditions, NRF2 is bound to its cysteine-rich partner, Kelch-like ECH-associated protein 1 (KEAP1), which represses NRF2 activity. Various phytochemicals, such as isothiocyanates, interact with KEAP1 which leads to the release of NRF2, resulting in its translocation to the nucleus and activation of ARE(Reference Yu and Kong83,Reference Dinkova-Kostova, Holtzclaw and Cole91) . Although dietary compounds and other chemicals that interact with KEAP1 and activate NRF2 have different molecular structures, they are all chemically reactive, and nearly all are electrophiles(Reference Dinkova-Kostova, Holtzclaw and Cole91). This allows them to react with SH groups of KEAP1, leading to the release of NRF2, which is now able to activate ARE. Such interaction with KEAP1 is not possible with the hydrophobic carotenoids, which generally lack any electrophilic group. Thus, it has been hypothesised that it is the carotenoid oxidation products that interact with KEAP1 and stimulate ARE. Linnewiel et al.(Reference Linnewiel, Ernst and Caris-Veyrat92) analysed the reactivity in ARE activation of a series of chemically synthesised apo-carotenoids that can potentially be derived from in vivo metabolism of carotenoids(Reference Kiefer, Hessel and Lampert93) or during their spontaneous or induced oxidation(Reference Caris-Veyrat, Schmid and Carail94). The activation of ARE by the synthetic apo-carotenals at concentrations of 10 µM correlated with the number of carbon atoms between the methyl group and the terminal carbonyl group. This number of carbon atoms determines the reactivity of the conjugated double bond in reactions such as Michael addition to thiol groups of proteins. A better description of the reactivity of the double bond is the characterisation by the electron density of the molecules. Indeed, in another study(Reference Linnewiel-Hermoni, Motro and Miller62), the electron density values of the apo-carotenals correlated with the inhibition of NF-κB transcriptional activity, suggesting that inhibition of NF-κB activity by carotenoids is also mediated by their derivatives. Other experiments in that study indicated that carotenoid derivatives could directly interact with two key proteins of the NF-κB pathway, probably through specific thiol groups of these proteins. It is possible that similar interactions occur between the apo-carotenals and the thiol groups of KEAP1. Although the above studies suggested that the active carotenoid derivatives are apo-carotenals, it was found that other derivatives such as apo-carotenoid acids and alcohols derived from lycopene can also activate ARE(Reference Lian and Wang95). However, when the reactivity of the three lycopenoids was compared via the induction of HO1 expression, it was found that the organic chemically synthesised potential human lycopene-metabolite apo-10′-lycopenal showed the strongest potential, suggesting that apo-carotenals are the most active mediators of NRF2 activation by carotenoids.
Carotenoids, carotenoid metabolites and retinoids as activators of nuclear hormone receptors (Fig. 2)

Fig. 2. Retinoids and carotenoid metabolites with known and potential nuclear hormone activation. Abbreviations: ATRA, all-trans retinoic acid; 9CRA, 9-cis-retinoic acid; 9DHCRA, 9-cis-13,14-dihydroretinoic acid; FAs, fatty acids; RAR, retinoic acid receptor; RXR, retinoid X receptor.
Regarding vitamin-A-like effects of carotenoids and carotenoid metabolites involving nuclear hormone receptors such as RARs and RXRs, the centric cleavage metabolites of β-carotene, the β-apo-15-carotenoids/retinoids, are further oxidised to the bioactive vitamin A derivatives, the apo-15-carotenoid acids, which are named retinoic acids(Reference Bohn, Desmarchelier and Sedef33,Reference Aydemir, Kasiri and Birta35,Reference Böhm, Lietz and Olmedilla-Alonso96) . These retinoic acids are well-known endogenous derivatives, functioning as lipid hormone ligands responsible for the activation of two major families of nuclear hormone receptors(Reference Dulińska-Litewka, Sharoni and Hałubiec36,Reference Heyman, Mangelsdorf and Dyck97–Reference Dulińska-Litewka, Hałubiec and Łazarczyk101) . These nuclear hormone receptors further, after ligand activation, directly interact with the genome and modify transcription of receptor-specific genes(Reference Balmer and Blomhoff102). The most abundant retinoic acid, i.e. all-trans-retinoic acid (ATRA), is the endogenous ligand of the RARs (RARα, β, γ):(Reference Petkovich, Brand and Krust99,Reference Mangelsdorf, Ong and Dyck100) . Besides ATRA, various other geometric isomers have been identified and are endogenously present, such as 13-cis-, 9,13-dicis-, 11-cis- and 9-cis-retinoic acid(Reference Sass and Nau103–Reference Kane, Chen and Sparks105). Besides ATRA, a large focus was also placed on 9-cis retinoic acid (9CRA), which was postulated to be the endogenous ligand of the RXRs (RXRα, β, γ)(Reference Heyman, Mangelsdorf and Dyck97,Reference Mangelsdorf, Borgmeyer and Heyman98) . Unfortunately, this claim is seen as highly controversial(Reference Rühl, Krezel and de Lera106), and its endogenous presence and its function as a physiologically relevant lipid hormone could not be conclusively confirmed by many groups working in the field of ultra-sensitive lipidomics (reviewed in refs.(Reference Rühl, Krezel and de Lera106–Reference De Lera, Krezel and Rühl108)). Alternatively, endogenously present geometric isomers of retinoic acid such as 13-cis-, 9,13-dicis- and 11-cis-retinoic acid were not described to be relevant for transmitting major biological activity via nuclear hormone receptor mediated signalling(Reference Allenby, Bocquel and Saunders109).
Various endogenous retinoids with a non-conclusively proven endogenous relevance were identified. These ranged from the phase 1 and deactivating metabolites, the hydroxyl- or oxo-metabolites of retinoic acids(Reference Frolik110), and various apo-carotenoids including apo-14′-carotenoic acid(Reference Aydemir, Dominguez and De Lera111,Reference Eroglu and Harrison112) apo-12′-carotenoic acid(Reference Eroglu, Hruszkewycz and Curley113), apo-13′-carotenone(Reference Aydemir, Dominguez and De Lera111,Reference Eroglu, Hruszkewycz and Curley113–Reference Sun, Narayanasamy and Curley115) , potential apo-15-lycopenoic acid(Reference Caris-Veyrat, Garcia and Reynaud34,Reference Aydemir, Kasiri and Birta35,Reference Narayanasamy, Sun and Pavlovicz116) and apo-10′-lycopenoic acid(Reference Gouranton, Aydemir and Reynaud78,Reference Ip, Liu and Lichtenstein117,Reference Ip, Hu and Liu118) , originating either from BCO1/BCO2-mediated cleavage(Reference Amengual, Widjaja-Adhi and Rodriguez-Santiago119–Reference Dela Seña, Sun and Narayanasamy121) or from an unspecific cleavage via radical-mediated pathways(Reference Caris-Veyrat, Schmid and Carail94,Reference Eroglu, Hruszkewycz and Curley113,Reference Yeum, Dos Anjos Ferreira and Smith122) . These derivatives were partly described as low-affinity ligands or as RAR/RXR antagonist, when the RAR or RXR is liganded by its endogenous ligand. The concept of an antagonist function of these apo-carotenoids has been described in various studies(Reference Eroglu, Hruszkewycz and Curley113,Reference Tang, Wang and Russell123) . Unfortunately, the endogenous relevance, starting from endogenous RAR activation by ATRA, which is only occurring in a short temporal and spatially restricted manner, was never clearly shown. In addition, the endogenous concentrations of these apo-carotenoids necessary at much higher levels, especially in the nucleus, where the interaction with the nuclear receptors takes place, to compete with and block ATRA-RAR-mediated signalling under physiological and nutritional conditions, were never reported, and their activity remains therefore speculative and was only shown under artificial conditions.
Recently, also dihydro-metabolites of apo-15-carotenoids were described to be present endogenously, ranging from 13,14-dihydroretinol(Reference Moise, Isken and Domiguez124–Reference Moise, Alvarez and Dominguez127) to the additional endogenously present all-trans- and 9-cis-13,14-dihydroretinoic acids (ATDHRA/9CDHRA)(Reference Moise, Kuksa and Blaner126,Reference Rühl, Krezel and de Lera128) , which were described as major relevant endogenous RAR as well as RXR ligands,(Reference Moise, Kuksa and Blaner126–Reference Rühl, Krezel and de Lera128)
The remaining question for alternative carotenoid metabolites functioning as nuclear hormone receptor ligands is the endogenous and nutritional relevance of these alternative ligands besides ATRA and 9CDHRA for RAR and RXR activation in mammalian organisms(Reference Krężel, Rühl and de Lera107,Reference De Lera, Krezel and Rühl108) . We can only report the perspective of presently known and potential endogenous carotenoid metabolites ‘here and now’ (Fig. 2), that oxo-/hydroxyl-retinoic acids(Reference Baron, Heise and Blaner129), apo-carotenoic acids(Reference Heyman, Mangelsdorf and Dyck97,Reference Petkovich, Brand and Krust99,Reference Aydemir, Dominguez and De Lera111,Reference Eroglu, Hruszkewycz and Curley113) apo-carotenons(Reference Eroglu, Hruszkewycz and Curley113) and apo-lycopenoic acids(Reference Aydemir, Kasiri and Birta35,Reference Narayanasamy, Sun and Pavlovicz116) might be of relevance for functioning as low-affinity ligands or, less likely, as RARs(Reference Eroglu, Hruszkewycz and Curley113) and RXRs(Reference Eroglu, Hruszkewycz and Curley113) under certain still non-identified physiological and nutritional conditions. In summary, there are many uncertainties but many options that, besides ATRA and 9CDHRA, multiple other carotenoid metabolites might be of major physiological and nutritional relevance for functioning as RAR and RXR ligands. Interferences in RAR- and RXR-mediated signalling might explain a large majority of carotenoid-mediated effects in a physiological and nutritional relevant range. Additional knowledge of these new pathways via novel carotenoid metabolites might result in more detailed insights about beneficial effects of carotenoids and in optimised suggestions for dietary intakes.
Regarding other nuclear hormone receptor mediated pathways, the RXR is the central heterodimer binding partner that interacts with the RAR or alternative nuclear hormone receptors such as the PPARs(Reference Chandra, Huang and Hamuro130) and alternative RXR-interacting heterodimer binding partners(Reference Mangelsdorf and Evans131). Indeed, PPAR-mediated effects were observed in white adipose tissue (WAT) of mice of BCO1−/− mice supplemented with β-carotene(Reference Amengual, Gouranton and van Helden132). Recently, also carotenoid metabolites were proposed to be potential endogenous physiologically and nutritionally relevant PPAR ligands(Reference Caris-Veyrat, Garcia and Reynaud34,Reference Cheng, Miao and Hu133) , which was already predicted by other studies(Reference Zaripheh, Nara and Nakamura134). Unfortunately, the bottleneck is that these carotenoid metabolites were not conclusively identified, neither isolated nor chemically synthesised, not further tested in molecular biological assays for their biological function and, finally, not identified endogenously to claim and confirm physiological or nutritional relevance(Reference Caris-Veyrat, Garcia and Reynaud34,Reference Cheng, Miao and Hu133) . Therefore, this topic of carotenoid metabolites functioning as physiologically or nutritionally relevant PPAR ligands remains speculative.
Additionally, there were interactions predicted of RAR, RXR with signalling pathways mediated by sex steroid mediated signalling such as androgen receptor mediated signalling(Reference Chuang, Lee and Lin135–Reference Murthy, Marcelli and Weigel138) as well as estrogen receptor mediated signalling(Reference Rühl, Fritzsche and Vermot139–Reference Murtaugh, Ma and Benson143). These interactions are of major importance for sex-specific diseases, including obesity/diabetes(Reference Ter Horst, Van den Munckhof and Schraa144) and cancer of exclusive male or female relevance, for instance, prostate cancer and breast/cervix cancers(Reference Mattiuzzi and Lippi145). This regulation can occur on multiple levels, involving retinoid signalling such as controlling of transcriptional expression of retinoid receptors(Reference Dulińska, Gil and Zagajewski146,Reference Dulińska-Litewka, Schmitz and Dembińska-Kieć147) , degrading metabolising enzymes(Reference Fritzsche, Vermot and Neumann141) and RA-synthesising enzymes(Reference Vermot, Fraulob and Dolle140).
Further signalling involving alternative nuclear hormone receptors, such as the constitutive androgen receptor mediated signalling(Reference Dawson and Xia148) and pregnane X receptor mediated signalling pathways(Reference Rühl149,Reference Rühl, Sczech and Landes150) , may also be of relevance.
Summary and evaluation of carotenoid-mediated mechanisms of action
Antioxidant effects of carotenoids have been well described in plants as well as in a large number of in vitro and in vivo experiments with animals and human relevance. Exposure to high concentrations, typically ranging around 10 to over 100 µM in in vitro experiments, as well as high nutritional dosages in human in vivo intervention trials (approximately 5–120 mg/d) have shown potential antioxidant activities, as shown by decreased markers of oxidative stress in mid-to-long-term human studies(Reference Bohn, Desmarchelier and Sedef33,Reference Böhm, Lietz and Olmedilla-Alonso151) , proposing protective effects of carotenoids on e.g. cellular membranes and lipoprotein particles. Whether these effects are based primarily on direct antioxidant effects or are transmitted also via interactions with transcription factors such as NRF2 and NF-κB is rather unclear.
However, concentrations at lower and more human-relevant, likely, physiological carotenoid levels, i.e. below or approximately 100 nM/0.1 µM in vitro, or even the lower concentrations of potential active apo-carotenoids (˜1–10 nM)(Reference Cooperstone, Novotny and Riedl152) have not clearly resulted in positive antioxidant effects, and thus their physiological relevance under such conditions may be questioned(Reference Erdman, Ford and Lindshield26). It is possible that they play more prominent roles when present at high concentrations in certain parts of the body, such as during digestion, although relevant studies on this topic are missing.
Nuclear hormone receptor activation, especially by apo-15-carotenoids, which are present in the human organism at ranges of 0.1–100 nM and are also termed retinoids, transmit their activity in the 1–10 nM range, and thus, human relevance seems likely(Reference Schmidt, Brouwer and Nau153). An overlap between in vitro and in vivo studies and human-relevant targets was clearly described and is reviewed in ref.(Reference Böhm, Lietz and Olmedilla-Alonso96).
Promising nutri-therapeutic effects were predicted by open-chain carotenoids and mainly lycopene in male-specific cancer symptoms mainly present in the prostate(Reference Holzapfel, Holzapfel and Champ154). While the lycopene-mediated signalling effects via RAR-mediated signalling pathways were predicted(Reference Caris-Veyrat, Garcia and Reynaud34,Reference Aydemir, Kasiri and Birta35,Reference Aydemir, Kasiri and Bartok155–Reference Ben-Dor, Nahum and Danilenko158) , still no enabling lycopene metabolites were found and identified(Reference Applegate, Rowles and Erdman159), and likely novel still non-identified signalling pathways interacting with androgen receptor and estrogen receptor signalling pathways may be of further relevance.
Bioavailability of carotenoids and alternative mechanisms of carotenoids
Bioavailability of carotenoids
Carotenoids are very non-polar molecules, which are grouped in two classes, i.e., the more polar carotenoids termed xanthophylls and non-polar hydrocarbons carotenoids referred to as carotenes and requiring micellisation, i.e. incorporation into mixed micelles prior to their absorption from the small intestine. As this process requires the presence of lipids, bile salts, digestive enzymes and a certain peristalsis, this process and resulting bioavailability are limited. Typically, only approximately 5–50 % of carotenoids are absorbed and reach the circulatory system, depending on the type of carotenoid, its polarity, and food matrix, but also host-related factors(Reference Reboul160,Reference Borel, Grolier and Mekki161) . More specifically, the more polar xanthophylls such as lutein are generally better absorbed than the apolar carotenes such as lycopene(Reference Meléndez-Martínez162). Also, the apparent chain length could play a role, with cis-carotenoids showing often a higher micellisation. Regarding the food matrix, liquid meals or heat-treated matrices resulting in macerisation of the plant cell wall generally result in higher bioavailability due to faster release kinetics(Reference Desmarchelier and Borel163). In addition, meals containing certain amounts of lipids(Reference Unlu, Bohn and Clinton164), limited amounts of dietary fibre(Reference Riedl, Linseisen and Hoffmann165) and perhaps limited amounts of minerals(Reference Borel, Desmarchelier and Dumont166,Reference Biehler, Hoffmann and Krause167) may foster carotenoid bioavailability. Among the host factors, diseases resulting in reduced absorbable surface in the intestine, possibly age, hormonal status and certain single-nucleotide polymorphisms of genes participating in cellular uptake or transport(Reference Bohn, Desmarchelier and Dragsted168) have been related to altered carotenoid bioavailability, among others.
Once absorbed, carotenoids are transported via lipoproteins to various tissues, and compartmental models have proposed various half-lives for different compartments, ranging from 2–7 d in plasma to 27–76 d in slow-exchanging tissues, likely adipocytes or muscle cells(Reference Bohn, Desmarchelier and Dragsted168). The excretion route may be via losses in bile or digestive juices, and also shorter apo-carotenoids or their resulting glucuronidated metabolites via the urine(Reference Bohn, Desmarchelier and Dragsted168) The predominant carotenoids reflect to a large extent those consumed via the diet, being namely β-carotene, lycopene, lutein, β-cryptoxanthin, α-carotene, zeaxanthin, phytofluene and phytoene, though not necessarily in this order(Reference Böhm, Lietz and Olmedilla-Alonso96). Thus, bioavailability is highly variable, not only between subjects but even within subjects, impeding clear cause-and-effect estimates.
Disease-related mechanisms potentially modulated via carotenoid–gut microbiota interaction
An altered gut microbiota has been correlated to a large number of diseases, including cardio-metabolic diseases and metabolic syndrome(Reference Wutthi-in, Cheevadhanarak and Yasom169) but also cancer(Reference Cheng, Wu and Yu170) and neurodegenerative diseases(Reference Tilocca, Pieroni and Soggiu171). Although very often a decreased microbial diversity is found in pathological conditions, the actual relationships between gut microbiome and diseases are still under evaluation. Recently, it was reported that, analysing available literature from 28 case–control studies, patterns of disease-related changes could be different with more or less specific shifts in the human gut microbiome(Reference Duvallet, Gibbons and Gurry172).
The mutual interaction between carotenoids or their derived products and the gut microbiota is still largely undefined since only a few studies have addressed this fundamental research question. It is, however, reasonable that, as a large amount of the carotenoids introduced through the diet remain unabsorbed, these compounds should reach the colon, becoming substrates for more or less extensive metabolism in this site, likely mediated by the gut microbiota(Reference Dingeo, Brito and Samouda173). It is known that a large amount of carotenoids are indeed fermented by the microbiota of the colon, into yet unknown products, following recovery fermentations ex vivo (Reference Grolier, Borel and Duszka174,Reference Kaulmann, André and Schneider175) . However, microbe-mediated carotenoid metabolism has not been thoroughly investigated. Recently, some interesting results were obtained. Most notably, in a recent intervention trial, the effect of lycopene (7 and 30 mg/d, for 1 month) on microbiota was investigated in 30 obese subjects, by providing a diet rich in tomato products or supplements(Reference Wiese, Bashmakov and Chalyk176). Lycopene showed dose-dependent increases of the relative abundance of e.g. Bifodobacterium adolescentis and B. longum. In addition, dose-dependent favourable reductions of LDL-C, LDL-peroxidase, and MDA/thiobarbituric acid reactive substances (TBARS) as markers of oxidative stress were reported. Djuric et al.(Reference Djuric, Bassis and Plegue177) studied the association between the microbiota composition of the colon mucosa and the serum carotenoid concentrations in subjects at increased risk for colon cancer. When stratifying subjects into tertiles of serum carotenoid levels, these authors found 11 operational taxonomic units associated with higher carotenoid levels, in particular a lower abundance of Firmicutes taxa (above all, the Lachnospiraceae family). It is also relevant to note that 36 % of the interindividual variance in serum carotenoid levels was explained by dietary intake, BMI, cholesterol levels, smoking and the relative abundance of Bacteroides, Roseburia and a genus of the Lachnospiraceae family. It should be mentioned that directing subjects towards a Mediterranean diet or Healthy Eating Diet for 6 months was not sufficient to change the colonic mucosal bacterial community, despite the observed increase in carotenoid intake.
Further contribution to the discussion on carotenoid–microbiota interaction comes from the study by Karlsson et al.(Reference Karlsson, Fak and Nookaew178), who tried to understand potential associations between symptomatic atherosclerosis and gut metagenome since bacteria are considered critical factors affecting the inflammatory status of the arterial wall. An increase of genes encoding peptidoglycan synthesis and reduction of genes encoding for phytoene dehydrogenase (precursor of both lycopene and β-carotene) were found in patients with respect to controls, together with reduced levels of serum β-carotene but not lycopene. These data seem to support a potential contribution of the microbiota to the carotenoid status, even if they could not prove any direct causal relationship.
Lyu et al.(Reference Lyu, Wu, Wang, Shen and Lin179), in a recent report on the effect of supplementation of β-carotene oxygenase (BCO2) knock-out (KO) mice with astaxanthin for 8 weeks, found an increased abundance of caecal Bifidobacterium spp. and higher levels of Proteobacteria and Bacteroides spp. in KO with respect to wild-type mice. The authors, based on this pilot study, suggested that both astaxanthin and BCO2 might affect gut microbiome. In this regard, the authors suggested that dietary carotenoids (e.g. β-carotene and astaxanthin) could promote gut heath through modulation of gut immune system maturation and immunoglobulin A production, likely regulating microbial dysbiosis. According to Liu et al.(Reference Liu, Liu and Fu180), an improved microbiota with an increase in Akkermansia spp. was found in a mouse model of alcoholic liver disease fed with a high-fat liquid diet and supplemented with astaxanthin (50 mg/kg body weight) for 12 weeks. Based on this promising result, this carotenoid was suggested by the authors as a potential candidate for the treatment of bacterial disorders found in alcoholic fatty liver disease. Similarly, a modulatory effect on lipid metabolism and gut microbiota was detected in mice fed with a high-fat diet supplemented with astaxanthin (0.005 % or 0.01 %) for 8 weeks(Reference Wang, Liu and Wang181).
Altogether, the data available appear too preliminary to demonstrate a direct involvement of carotenoids in the modulation of microbiota, and as far as provitamin A carotenoids are concerned, the impact on vitamin A status could be also a relevant mechanism to consider. In fact, vitamin A has been reported to play a role in the balance of the intestinal barrier(Reference Lyu, Wu, Wang, Shen and Lin179), and the gut microbiota was altered by a vitamin-A-deficient diet in rats and mice(Reference Amit-Romach, Uni and Cheled182,Reference Cha, Chang and Chang183) . Nevertheless, a targeted analysis of microbiota-derived metabolising activities would be pivotal for the identification of the existence of a carotenoid–microbiota–host interaction.
Health-beneficial effects mediated by carotenoids
Many health-beneficial effects of carotenoids have been described, and were mainly associated with high carotenoid levels in blood as well as in organs(Reference Bonet, Canas and Ribot38,Reference Agarwal and Rao184) . The connection to a reduced intake of fruits and vegetables, which are high in carotenoids, was rapidly drawn. However, fruits and vegetables are also rich in a large variety of additional health-beneficial organic compounds, including polyphenols, antioxidant vitamins C and E, and dietary fibre, among others(Reference Pandey and Rizvi185). A clear distinction of health-beneficial effects dedicated to one single carotenoid or even one single carotenoid metabolite with one mechanistic target has been aimed for, but has thus far not been clearly shown. It seems more likely that multiple carotenoids, with multiple carotenoid metabolites, acting via multiple mechanistic pathways in concert are responsible for the observed actions. Here, we try to describe, based on obesity and skin/lung cancer, the current situation and multiple mechanisms identified in experimental studies. A targeted supplementation with carotenoids, aiming at better health, is preferred for specific diseases such as of the macula, while, alternatively, a healthy diet rich in fruits and vegetables is likewise recommended but unfortunately not well accepted by a convenience-lifestyle-driven general population.
Carotenoids and obesity
In recent years, a novel perspective on the function and health benefits of carotenoids and carotenoid-derived products is emerging that connects these compounds to lipid and energy metabolism in homeostatic tissues, decreased body fat accumulation and the control of adipocyte biology, with possible implications for the aetiology and management of obesity and obesity-related metabolic disorders such as insulin resistance, diabetes and cardiovascular disease(Reference Bonet, Ribot and Felipe186–Reference Bonet, Ribot and Galmés192).
Adipose tissue, in addition to the liver, is an important site of carotenoid and retinol storage/accumulation(Reference Kaplan, Lau and Stein193–Reference Tsutsumi, Okuno and Tannous195). It has been estimated that 15–20 % of total body retinol in rats is stored in adipose tissue, in particular in the adipocytes(Reference Tsutsumi, Okuno and Tannous195). Carotenoids are found in adipocytes mainly in the lipid droplets, and also in association with cell membranes(Reference Gouranton, Yazidi and Cardinault196). Carotenoid concentrations in abdominal fat depots show a strong association with both dietary carotenoid intake and plasma carotenoid concentrations in humans(Reference Kohlmeier and Kohlmeier197–Reference El-Sohemy, Baylin and Kabagambe199). Provitamin A carotenoids and retinol in adipocytes may serve to regulate systemic vitamin A homeostasis since adipose tissue produces retinol-binding protein and adipose retinol/retinyl esters stores are readily mobilised under conditions of dietary vitamin A deficiency(Reference Frey and Vogel200). Additionally, different lines of evidence support specific activities of carotenoids and retinoids in adipose tissue and adipocyte biology (see below).
Adipose tissue expresses all intracellular binding proteins, enzymes and transcription factors involved in carotenoid and retinoid metabolism and function, including the carotenoid cleavage enzymes BCO1 and BCO2(Reference Tourniaire, Gouranton and Von Lintig189–Reference Hessel, Eichingr and Isken201). Retinal and ATRA have been detected in adipose tissue, in the case of ATRA at relatively high levels compared with other tissues(Reference Kane, Folias and Napoli202–Reference Perumal, Sriram and Lim204). Studies suggest a crosstalk of intracellular retinoid metabolism and lipid droplet dynamics, with a physical association of enzymes of retinoid metabolism with the lipid droplet coat that appears to depend on active acyl ester biosynthesis(Reference Jiang and Napoli205,Reference Jiang and Napoli206) . Animal(Reference Sima, Manolescu and Bhat207) and human(Reference Gerhard, Styer and Strodel208–Reference Peinado, Jimenez-Gomez and Pulido211) studies have revealed a differential expression of genes for carotenoid/retinoid-metabolising enzymes in visceral and subcutaneous adipose tissues, which display important differences regarding developmental origin, metabolism, endocrinology, capacity for adipogenesis and the health risk they entail(212,Reference Lee, Wu and Fried213) . Genetic ablation of different carotenoid/retinoid-metabolising enzymes and transport proteins results in alterations of adiposity and defects in brown adipose tissue (BAT) thermogenesis in mice(Reference Frey and Vogel200,Reference Krois, Vuckovic and Huang214,Reference Fenzl, Kulterer and Spirk215) Furthermore, adipocyte functions such as the thermogenic capacity of BAT in rodents are dependent on the animal’s vitamin A status(212,Reference Krois, Vuckovic and Huang214,Reference Felipe, Bonet and Ribot216) .
Accumulating evidence links carotenoids and carotenoid metabolites to the inhibition of adipocyte differentiation (adipogenesis) and the reduction of fat storage in mature adipocytes, through suppression of PPARγ, a master regulator of adipogenesis and the mature adipocyte phenotype. Carotenoids and carotenoid derivatives function in this sense either by acting possibly as PPARγ antagonist ligands – e.g. retinal(Reference Ziouzenkova, Orasanu and Sharlach217), apo-14′-carotenal(Reference Ziouzenkova, Orasanu and Sukhova218) and intact astaxanthin(Reference Inoue, Tanabe and Matsumoto219) – or by repressing PPARγ expression secondarily to RAR activation – e.g. ATRA(Reference Xue, Schwarz and Chawla220), intact β-carotene(Reference Lobo, Amengual and Li221) and β-cryptoxanthin(Reference Shirakura, Takayanagi and Mukai222). Among other mechanisms, interference with C/EBP action on the PPARγ promoter by liganded RAR is an important contributor to ATRA-dependent inhibition of adipogenesis(Reference Schwarz, Reginato and Shao223). How retinoid-activated RAR inhibits PPARγ in the mature adipocyte is not known, but mechanisms similar to those involved in the inhibition of adipogenesis may be involved.
Importantly, carotenoids and carotenoid-derived products with anti-adiposity action, besides antagonising or repressing PPARγ, are able to promote lipid oxidation in adipose and other tissues. Dietary supplementation with carotenoids such as β-carotene, fucoxanthin, astaxanthin, crocetin and β-cryptoxanthin – among others – or treatment with retinoids such as ATRA and retinal enhance systemic fat catabolism and energy expenditure in rodents, resulting in an anti-obesity action which is not due to reduced food intake(Reference Bonet, Ribot and Palou187,Reference Luisa Bonet, Canas and Ribot188,Reference Bonet, Ribot and Galmés192) . Focusing on adipose tissues, early studies showed that ATRA, as well as β-carotene and other provitamin A carotenoids, increases uncoupling protein 1 (UCP1) and, thus, the capacity for energy expenditure through enhanced BAT thermogenic function(Reference Bonet, Ribot and Felipe186). More recently, fucoxanthin(Reference Maeda, Hosokawa and Sashima224), β-cryptoxanthin(Reference Hara, Takahashi and Mohri225), lycopene(Reference Wang, Suo and Zhang226), ATRA(Reference Mercader, Ribot and Murano227,Reference Tourniaire, Musinovic and Gouranton228) and retinal(Reference Kiefer, Vernochet and O’Brien209) have been shown to stimulate WAT oxidative capacity and features of WAT browning, which may also contribute to greater energy expenditure. Effects in adipose tissues most likely result from the combination of direct action of the carotenoids/retinoids in these tissues and their systemic effects. For instance, ATRA treatment in mice induces UCP1 at the transcriptional level in adipocytes and mobilises the browning-inducing myokine irisin from skeletal muscle(Reference Amengual, García-Carrizo and Arreguín229).
Mechanisms of gene expression regulation are better known for ATRA. Several genes for proteins in energy and lipid catabolism are up-regulated at the transcriptional level, following ATRA binding to the canonical RAR moiety (or the PPARβ/δ moiety) of RXR heterodimers and subsequent recruitment of cofactor complexes on the target gene promoter(Reference Bonet, Ribot and Palou187). In addition, some RXR heterodimers (so-called permissive) respond to ligands of either partner and are synergistically activated when both ligands are bound, providing a mechanism for widespread effects of retinoids on gene expression(Reference Aranda and Pascual230). Notably, liver X receptor (LXR) and PPAR isoforms, which are deeply involved in the control of different aspects of lipid metabolism, act on (at least some) target genes as permissive heterodimers with RXR(Reference Willy, Umesono and Ong231,Reference Mukherjee, Davies and Crombie232) . Finally, retinoids may impact cell metabolism through extragenomic actions, such as retinoylation (acylation by retinoic acid) of proteins and, especially, the activation of protein kinase cascades such as p38 mitogen-activated protein kinase (P38 MAPK or MAPK14) or AMP-activated protein kinase (AMPK)(Reference Bonet, Canas and Ribot38,Reference Bonet, Ribot and Palou187,Reference Luisa Bonet, Canas and Ribot188) .
Closely related to its metabolic role, adipose tissue has an important endocrine function: it produces and secretes many signalling molecules including proteins (collectively named adipokines), as well as immunomodulatory factors whose altered production in obesity links visceral obesity to associated metabolic disturbances(Reference Wisse233). By altering the endocrine function of adipose tissues, carotenoids and their conversion products may elicit adipose-driven effects in distant organs protective against obesity and/or its clinical complications. Carotenoids and their conversion products affect the secretory profile of adipose tissue by affecting the activity of target transcription factors and inflammatory pathways, and possibly also in part in a passive manner, i.e. secondary to effects on adipocyte lipid content and body fat. Studies have shown that β-carotene and ATRA can actively suppress adipose production of leptin, resistin and retinal-binding protein 4 (RBP4) – three adipokines for which elevated serum levels associate with inflammation and insulin resistance in humans and rodents(Reference Ouchi, Parker and Lugus234) – whereas capsanthin/capsorubin, crocetin, fucoxanthin, lycopene and β-carotene (but not ATRA) can up-regulate adiponectin – an adipokine that is down-regulated in obesity and with well-established insulin-sensitising, anti-inflammatory and anti-atherogenic action(Reference Turer and Scherer235) (reviewed in refs.(Reference Bonet, Canas and Ribot38,Reference Bonet, Ribot and Palou187,Reference Luisa Bonet, Canas and Ribot188,Reference Bonet, Ribot and Galmés192) ).
Anti-inflammatory action of carotenoids in adipose tissue may relate to carotenoids’ ability to reduce oxidative stress, considering that excess ROS production in obese adipose tissue is a pro-inflammatory, pathogenic mechanism of obesity-associated metabolic syndrome(Reference Le Lay, Simard and Martinez236). Antioxidant properties of carotenoids stem from their scavenging function toward reactive species and, especially, their ability to activate the NRF2 pathway and, hence, cellular antioxidant defences, and to suppress the NF-κB pathway, thus inhibiting downstream production of inflammatory cytokines(Reference Kaulmann and Bohn28,Reference Bonet, Canas and Ribot38,Reference Bonet, Ribot and Palou187,Reference Luisa Bonet, Canas and Ribot188) . At the adipocyte level, antioxidant action has been evidenced for β-carotene, astaxanthin, fucoxanthin and crocetin and anti-inflammatory action for lycopene and fucoxanthin, and its potential metabolites apo-10′-lycopenoic acid and fucoxanthinol, as well as ATRA(Reference Karkeni, Bonnet and Astier237). Nevertheless, under certain conditions, carotenoids may be pro-oxidants in cells, when highly reactive oxidative breakdown products accumulate(Reference Ren, Yang and Zhang238) or when carotenoid detoxifying mechanisms are compromised(Reference Amengual, Lobo and Golczak239).
In humans, epidemiological studies have consistently reported decreased serum levels of carotenoids, including β-carotene in overweight and obese individuals, both adults and children/adolescents, and an inverse association between circulating carotenoid concentrations and measures of obesity, such as BMI or waist circumference, and of obesity-related metabolic disorders(Reference Harari, Coster and Jenkins240) (reviewed in ref.(Reference Luisa Bonet, Canas and Ribot188)). The inverse association of serum total and individual carotenoids levels with metabolic syndrome is confirmed in a recent meta-analysis(Reference Beydoun, Chen and Jha241). The concentration of carotenoids in adipose tissue(Reference Luisa Bonet, Canas and Ribot188) and isolated adipocytes(Reference Östh, Öst and Kjolhede242) is also lower in obese people. In addition, obese individuals showed a reduced efficiency of β-carotene conversion to retinoids(Reference Wise, Kaats and Preuss243). In a recent prospective study, circulating ATRA levels at baseline predicted the development of metabolic syndrome at 4-year follow-up(Reference Liu, Chen and Mu244). However, an association of vitamin A status as serum retinol levels with human obesity or metabolic syndrome is less clear(Reference Luisa Bonet, Canas and Ribot188,Reference Harari, Coster and Jenkins240,Reference Beydoun, Chen and Jha241) . Studies have also pointed to an association between higher dietary intakes of carotenoids (as evaluated through food frequency questionnaires) and reduced adiposity and development of obesity-related metabolic diseases(Reference Sluijs, Beulens and Grobbee245,Reference Kesse-Guyot, Ahluwalia and Lassale246) . Despite the epidemiological evidence, intervention trials to assess carotenoids in relation to adiposity in humans are scarce. Nevertheless, beneficial effects on adiposity in overweight and obese humans have been achieved through supplementation of carotenoid mixtures (either pure or in the form of plant juices or extracts), at doses lower than those that caused concern and controversy in the past in large-scale β-carotene intervention trials(Reference Luisa Bonet, Canas and Ribot188,Reference Canas, Lochrie and McGowan247,Reference Kakutani, Hokari and Nishino248) . Evidence from intervention studies of anti-obesity action is mainly related to β-carotene, β-cryptoxanthin, fucoxanthin and paprika xanthophylls.
Taken together, many cell culture and animal studies indicate that specific carotenoids and carotenoid derivatives impact in direct and indirect manners essential aspects of adipose tissue biology, including the control of adipogenesis, adipocyte metabolism (relative capacities for fat storage and oxidation), the production of regulatory signals and inflammatory mediators, and oxidative stress. These aspects may be of special relevance in regard to obesity, as obesity entails inflammation of adipose tissue(Reference Wisse233) and the development of hypertrophic adipocytes in which oxidative stress is exacerbated(Reference Le Lay, Simard and Martinez236). More human intervention and mechanistic studies are needed to verify the potential of specific carotenoids against obesity and ‘sick fat’ and to fully understand the connection of carotenoids with the control of adiposity.
Carotenoids and cancer
The potential of carotenoids to prevent the onset of certain cancers has been studied for many years(Reference Bolhassani249–Reference Nishino, Murakoshi and Ii251), but conclusive mechanisms of action are still not clearly known. Besides the antioxidant effects mainly found in experimental models that have never been conclusively confirmed in humans, the antioxidant/anti-inflammatory cascades involving the transcription factors NF-κB (RELA) and NRF2 (NLF2L2, section B2) and nuclear hormone receptor activation potential by mainly carotenoid metabolites (section B3) have been implicated. In addition, effects on cell cycle control, differentiation and apoptosis in RAR-, RXR- and partly PPAR-mediated pathways may be modified by retinoids as carotenoid metabolites(Reference Altucci and Gronemeyer252–Reference Klaassen and Braakhuis254). As cancer is a heterogeneous disease, in this review we focus on two types of cancer: skin and lung cancer.
Skin cancer and carotenoids
Skin cancers are one of the most common forms of human neoplasia and can be divided into two groups: melanoma cancer, with one of the highest rates of morbidity and mortality among all cancers(Reference Thompson, Surjana and Halliday255) and non-melanoma cancers (NMSC), including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), which are rarely lethal(Reference Berry256). In the development of skin cancer and skin aging, UV radiation is considered the most important environmental factor, indicating the need for some chemoprotective agents to slow down the increasing incidence(Reference Kim and He257).
One important mechanism for skin cancer prevention is the photo-protective effects of carotenoids. Carotenoids, especially β-carotene, lycopene, astaxanthin, canthaxanthin and lutein were investigated as photo-protective agents in several human intervention studies, mainly as agents which prevent solar erythema formation(Reference Stahl and Sies258) and photo-induced carcinogenesis, but also for photo-aging prevention(Reference Balić and Mokos259). Both, antioxidant mechanism as well as blue-light filtering properties of carotenoids(Reference Junghans, Sies and Stahl260), could play a role. Their role as provitamin A compounds, protective pigments, efficient antioxidants and anti-inflammatory agents makes them attractive candidates for skin protection. Consequently, carotenoids have been used as both oral supplements and as a component of topical sunscreen products.
UV radiation is associated with approximately 65 % of melanoma cases and 90 % of non-melanoma skin cancers(Reference Kim and He257) Despite the primary prevention measures, using sunscreen and wearing protective clothing are highly promoted(Reference Armstrong and Kricker261,Reference Thompson, Jolley and Marks262) . DNA damage induced by UVB (290–320 nm) radiation primarily leads to the formation of dimeric lesions, with the main dimeric photolesions being cyclobutane pyrimidine dimers and pyrimidine (6-4) photoproducts (6-4PPs)(Reference Balić and Mokos259,263,Reference De Gruijl, Van Kranen and Mullenders264) , DNA strand breaks, and DNA cross-links(Reference Kim and He257). Moreover, excessive exposure to UV induces mutations of P53 and loss of FAS–FASL interaction. Keratinocytes with these mutations become resistant to apoptotic pathways, and consequently, the expansion of these clones eventually leads to the formation of squamous cell carcinomas and/or actinic keratoses(Reference Melnikova and Ananthaswamy265). On the other hand, UVA (320–400 nm) radiation leads to oxidative damage in the cell with formation of ROS, oxidative stress and oxidation of nucleotide bases, the main lesion being 8-oxo-7,8-dihydroguanine(Reference Thompson, Surjana and Halliday255). ROS affect several signalling pathways such as c-Jun N-terminal kinase (JNK or MAPK8), myelocytomatosis oncogene (MYC), Keap1, mitogen-activated protein kinases (MAPKs), NRF2, MAPK14, P53, protein kinase C, RAS, RAF, and even more(Reference Zhang, Wang and Vikash266).
Sunscreen products are designed to protect the skin from the harmful effects of UV wavelengths of sunlight, namely UVB (280–315 nm) and UVA (315–400 nm), that reach the Earth’s surface(Reference Paul and Gwynn-Jones267). UVB can damage the DNA directly, while UVA acts mainly through ROS intermediates(Reference Chen, Hu and Wang268). Chromophores in the skin, including melanin, porphyrins, heme, cytochromes and riboflavin, can absorb UVA photons. Singlet oxygen and other ROS are formed if this absorbed energy is subsequently transferred to oxygen. Environmental pollutants, including polycyclic aromatic hydrocarbons, can act synergistically with UVA to increase the production of superoxide and singlet oxygen. Overexposure to UV radiation contributes to the development of skin cancers and skin ageing(Reference Chen, Hu and Wang268). Therefore, many cosmetic products (sunscreen lotions, creams, shampoo, hair dyes and varnishes, lipsticks, etc.) contain different UV filters to either reflect or absorb UV light(Reference Silvia Díaz-Cruz, Llorca and Barceló269,Reference Serpone, Dondi and Albini270) . Sunscreens on the market tend to protect better against UVB than UVA radiation. Haywood and co-workers have reported that sunscreens with broad-spectrum UV protection reduce free radical formation by 55 %(Reference Haywood, Wardman and Sanders271). Carotenoids may act as accessory pigments and provide photo-protection by dissipation of excess light energy by quenching the excited triplet state molecules and singlet oxygen, Therefore, their addition complementing the protection of UV filters may represent an added value to sunscreens(Reference Demmig-Adams and Adams272). Another strategy may be the activation antioxidative mechanisms(Reference Poljsak, Šuput and Milisav273). This is hinted at by the observation that moderate physical activity, inducing mild oxidative stress, may increase skin tolerance to harmful levels of oxidative stress(Reference Kruk and Duchnik274).
Sunscreens are one of the most widely applied strategies by the public to protect themselves from UV irradiation; nevertheless, topical treatments with antioxidants, especially carotenoids, are less tested than oral supplementation(Reference Commander, Chang and Fakhro275). The combination of oral and topical lutein and zeaxanthin treatments provided better antioxidant protection than either treatment alone; the least effective was the topical treatment alone, as it was inferior even to oral carotenoid administration(Reference Palombo, Fabrizi and Ruocco276). β-Carotene is a major constituent of commercially available oral sun protectants, but studies proving protective effect of oral and topical treatment with β-carotene against skin responses to sun exposure are scarce and conflicting(Reference Stahl, Hasan and Farnukh277). Short-term oral supplementation of carotenoids resulted in a range of outcomes, from protection against photo-damage, to no protection observed, to possible pro-oxidative properties at higher dosages and synergistic effects between the antioxidants. Published successful studies are described in Table 1. The efficacy of β-carotene for systemic photo-protection seems to depend on the dose and duration of the treatment; it seems that at least 10 weeks of carotenoid treatment are needed for a measurable short-term improvement for systemic photo-protection against erythema formation(Reference Sies and Stahl278). Additionally, synergistic effects between different antioxidants are important to increase the protection against ROS. β-Carotene as well as other carotenoids, such as lutein and lycopene, can ameliorate UV-induced erythema in humans(Reference Heinrich, Gartner and Wiebusch279). The combination of carotenoids and other antioxidants is also effective, as was shown for the synergistic effects of other groups of antioxidants. For example, ascorbate can regenerate α-tocopherol from its phenoxyl radical in many model systems(Reference Guo and Packer280), selenium and niacin are required to keep glutathione in its active form(Reference Kruk and Duchnik274,Reference John and Gutteridge281) and the vitamin C radical may be recycled by GSH non-enzymatically(Reference Stocker, Weidemann and Hunt282). The beneficial effects of combined antioxidants were reported by Cesarini et al.(Reference Césarini, Michel and Maurette283), based on a study on 25 healthy individuals receiving an antioxidant complex and vitamins (lycopene, β-carotene, α-tocopherol and selenium) that improved epidermal defence and reduced UV-induced damage. Gollnick et al.(Reference Gollnick, Hopfenmüller and Hemmes284) concluded that supplementation with moderate dosages of β-carotene (30 mg/d) before and during sunlight exposure can protect against sunburns, possibly because of increased absorption capacity of the skin or because β-carotene concentration in the skin does not decrease to below the concentration considered to be critical. On the other hand, post-supplementation with β-carotene may have detrimental effects, most probably due to its effect on accelerated cell proliferation(285).
Table 1. Effects of carotenoid supplementation for skin protection

Type of administration: O, oral; OT, oral and topical; T, topical.
While carotenoids have been shown to inhibit the UV-induced epidermal damage and tumor formation in mouse models(Reference Mathews-Roth and Krinsky286), these data were not reproduced in large long-term human intervention studies so far(Reference Kruk and Duchnik274). Therefore, oral supplementation of antioxidants, including carotenoids, without a specific medical diagnosis, is not recommended at the moment.
Interest in the use of natural products as a possible treatment option of skin cancer is growing, as 70 % of anti-cancer drugs are obtained from natural sources(Reference Mathews-Roth and Krinsky286). Carotenoids are promising anti-cancer agents and able to interfere with the cell cycle(Reference Nishino, Murakoshi and Ii251). Effects of carotenoid administration may differ, depending on the stage of malignant transformation. In normal cells, they may reduce the frequency of the malignant transformation by ROS-induced damage prevention or by induction of cellular repair and adaptive stress responses. Increased ROS formation can protect from cancer by increasing the oxidative stress/damage and eliminating the damaged cells(Reference Kirshner, He and Balasubramanyam287). The reduction of ROS by antioxidants can lead to the survival of preinitiated tumor cells even in unnatural matrix environments(Reference Schafer, Grassian and Song288). Likewise, the increased administration of antioxidants and possibly carotenoids during cancer promotion, progression and treatment phases may increase the cancer cell survival rate by enhanced resistance against oxidative stress and decreased apoptosis(Reference Watson289). Carotenoids may act as accessory pigments and provide photo-protection by dissipation of excess light energy and by quenching excited triplet state molecules and singlet oxygen(Reference Demmig-Adams and Adams272).
A likely more important mechanisms compared with simple low-efficient photo-protective effects of carotenoids, again, is the function of carotenoids to function as RAR-ligand precursors(Reference Rühl, Bub and Watzl290). ATRA, the active metabolite of provitamin A carotenoids, can interfere with RAR signalling, which is protective against cancer, especially skin cancer, with induction of apoptosis of cells which may be pre-cancerous(Reference Persaud, Park and Ishigami-Yuasa291). Skin and plasma carotenoid concentrations decrease with UV irradiation in human volunteers(Reference Biesalski, Hemmes and Hopfenmuller292). UV exposure has also been associated with local vitamin A deficiency which could be preventable by ATRA pre-treatment(Reference Wang, Boudjelal and Kang293) and likely by local and topical provitamin A pre-treatment – not to forget, the beneficial effect of ATRA and alternative other RAR activators, in skin cancer therapy(Reference Micali, Lacarrubba and Dinotta294,Reference Levine295) .
Lung cancer and carotenoids
β-Carotene is an effective antioxidant in vitro, and it has been hypothesised that it is also effective in vivo (Reference Schwartz, Antoniades and Zhao296). Indeed, epidemiological studies have suggested that high dietary β-carotene reduces the risk for several types of cancer, including lung cancer(Reference Ziegler297,Reference Peto, Doll and Buckley298) . Smokers and asbestos-exposed subjects have an increased lung cancer risk(Reference Brüske-Hohlfeld299), in combination with a lower β-carotene status(Reference Van Antwerpen, Theron and Richards300). The hypothesis that β-carotene has the ability to decrease lung cancer risk was tested in the Alpha-Tocopherol β-Carotene (ATBC) randomised control trial(Reference Albanes, Heinonen and Taylor301). In this study, smokers were supplemented with 20 mg β-carotene per day and/or vitamin E (50 mg/d). The study was performed in 29,133 male participants with a median duration of 6.1 years, resulting in an average β-carotene concentration of 5.59 µM β-carotene in blood after 3 years of intervention(Reference Albanes, Heinonen and Huttunen302). In the same period, this hypothesis was also tested in the Carotene and Retinol Efficacy (CARET) trial(Reference Omenn, Goodman and Thornquist303), which used doses of β-carotene and vitamin A several times above the normal, average level(Reference Böhm, Lietz and Olmedilla-Alonso151). Indeed, smokers and asbestos-exposed subjects were supplemented with 30 mg β-carotene and 25,000 IU retinyl palmitate per day. This CARET study was performed with 18,314 participants (males and post-menopausal females) with a median duration of 3.7 years, resulting in an unknown increase in β-carotene plasma concentration. The CARET and the ATBC studies surprisingly resulted in an increased, rather than a decreased, lung cancer risk(Reference Albanes, Heinonen and Taylor301,Reference Omenn, Goodman and Thornquist303) . The ATBC study also reported other detrimental effects, such as an increased risk for cardiovascular diseases(Reference Rapola, Virtamo and Ripatti304), and the CARET study reported an increased mortality due to coronary heart disease(Reference Omenn, Goodman and Thornquist13). The outcome contrasts with that of a study with 22,071 non-smoking male physicians, where the subjects were supplemented with 50 mg β-carotene every alternating day, resulting in 2.24 µM β-carotene in the blood. In this study, β-carotene supplementation did not result in any observed detrimental effects(Reference Hennekens, Buring and Manson305). The fact that the volunteers in this study were non-smoking, in contrast to the ATBC study and the CARET study, has been used as an explanation for the different outcome, but, for example, also the supplement intake regimen differed: every other day in this study, versus every day in the ATBC and CARET studies.
The ATBC and CARET studies have shed doubt on the use of β-carotene as a supplement and have resulted in cautionary advice of the EFSA to not consume more than 25 mg β-carotene per day, especially in combination with smoking(306). To be able to fully and safely exploit the beneficial effects that are associated with high dietary carotenoid intake, it is necessary to understand the mechanisms behind the observed adverse effects in the ATBC and CARET trials. Mechanistic studies are, however, hampered by the availability of animal models that reflect human β-carotene metabolism. In humans, a large percentage of β-carotene, 30–70 %, depending on genotype(Reference Borel and Desmarchelier307), is taken up intact. This is not the case in rodents, which are animal models well suited for mechanistic studies. Compared with humans, rodents have a more active variant of BCO1(Reference Von Lintig, Hessel and Isken308), the enzyme that centrally cleaves β-carotene. This happens already in the intestinal epithelial cells upon uptake, and hardly any β-carotene can be found in circulation. Since ferrets do have a β-carotene metabolism that resembles that of humans, ferrets have been used as an animal model for mechanistic studies into functional effects of β-carotene. However, due to a lack of molecular tools at the time, only few possible mechanisms of β-carotene action were identified. Most notably, physiological (0.43 mg/kg body weight/d) β-carotene concentrations resulted in an increase in ATRA concentrations in the lung in smoke-exposed ferrets, while pharmacological concentrations (2.4 mg/kg body weight/d) decreased ATRA concentrations and RAR β(Reference Liu, Russell and Wang309). Part of this effect has been explained by smoke-induced β-carotene oxidation. Vitamins E and C have been shown to decrease oxidative breakdown of β-carotene. Indeed, β-carotene supplementation in combination with these vitamins resulted in higher ATRA concentrations than after β-carotene supplementation alone(Reference Liu, Russell and Wang310). However, other mechanisms may have played a role. In inflammation, which is associated with smoking, plasma β-carotene concentrations decreased to a higher extent than the plasma concentrations of the more oxidation-sensitive carotenoids lycopene and lutein(Reference Liu, Russell and Wang310,Reference Erlinger, Guallar and Miller311) . Indeed, examination of deposited human microarray gene expression data showed that smoking results in increased expression of alcohol dehydrogenase 7 (ADH7)(Reference Carolan, Heguy and Harvey312–Reference Spira, Beane and Shah315). ADH7 is the most important enzyme in the conversion of retinol to retinal(Reference Yang, Davis and Hurley316), indicating that smoking not only imposes oxidative stress, but also affects vitamin A homeostasis. Moreover, higher protein levels of phosphorylated JNK, MAPK14 (P38) and Jun proto oncogene (JUN), were observed in groups exposed to smoke with higher, non-nutritionally relevant dose of β-carotene compared with smoke-exposed ferrets alone. On the other hand, physiological doses of β-carotene to smoke-exposed ferrets decreased phosphorylated levels of JNK, MAPK14 and JUN(Reference Liu, Russell and Wang317). JNK and MAPK14, members of the MAPK family, mediate cellular responses to cytokines and environmental stress and may play an important role in inflammation and carcinogenesis(Reference Wagner and Nebreda318). In another study performed in ferrets, β-carotene was used in combination with benzo[a]pyrene, the major carcinogenic compound from cigarette smoke. β-Carotene reduced C-centred radicals in vitro and in vivo, while OH-centred radicals were increased in vitro, but remained unaffected in vivo, likely due to up-regulation of DNA damage repair(Reference van Helden, Keijer and Heil27). No evidence for adverse effects was found in this study.
Mechanisms of β-carotene supplementation for cancer protection
Detailed mechanistic studies became possible owing to the availability of a mouse model in which BCO1 was inactivated (BCO1−/−)(Reference Hessel, Eichingr and Isken201). Like humans, BCO1−/− mice displayed increased plasma β-carotene upon β-carotene supplementation and accumulated β-carotene in the lung upon β-carotene supplementation(Reference Hessel, Eichingr and Isken201). To identify molecular effects of β-carotene, female and male BCO1−/− mice were supplemented with either 1500 IE vitamin A per kg diet, to assure vitamin A sufficiency, or with 1500 IE vitamin A plus 150 mg β-carotene per kg of diet, and whole genome microarray analysis was performed. Of interest, differences were observed in the response to β-carotene supplementation between male and female mice. In fact, only a limited number of genes were commonly regulated between female and male mice(Reference Van Helden, Godschalk and Swarts319). In total, 1522 genes were regulated by β-carotene in the lung of BCO1−/− females and 1474 in males(Reference Van Helden, Godschalk and Swarts319). Out of these, only 89 genes were affected in both sexes, of which the large majority, 85, were regulated in the opposite direction(Reference Van Helden, Godschalk and Swarts319). The functional responses also differed. BCO1 inactivation appeared to increase the requirement for vitamin A in females, which was alleviated by β-carotene supplementation, despite the absence of BCO1(Reference Van Helden, Heil and Van Schooten320). This effect on inflammation was not observed in male BCO1−/− mice(Reference Van Helden, Godschalk and Heil321). Rather, in the lung of BCO1−/− mice males, two genes were strongly down-regulated by β-carotene: frizzled class receptor 6 (FZD6) and collagen triple helix repeat containing 1 (CTHRC1)(Reference Van Helden, Godschalk and Swarts319), both genes having a role in developmental WNT signalling(Reference Dinkova-Kostova, Holtzclaw and Cole91,Reference Katoh322–Reference Jiang, Cui and Liu324) . Differences between males and females were also observed in the post-intervention period of the CARET study (the ATBC study concerned males only), with larger relative risks of lung cancer mortality (1.33 versus 1.14; P = 0.36), cardiovascular disease mortality (1.44 versus 0.93; P = 0.03), and all-cause mortality (1.37 versus 0.98; P = 0.001) in the post-menopausal females(Reference Goodman, Thornquist and Balmes325).
Do the mechanistic data explain the adverse effects? To clarify downstream effects of FZD6 down-regulation and assess human relevance, Fzd6 was inactivated, using RNAi, in BEAS2B cells, human type 2 bronchial epithelial cells, and whole-genome gene expression was analysed(Reference Piga, Van Dartel and Bunschoten326). The genes that were regulated both in vitro by FZD6 inactivation in BEAS2B cells and in vivo by BC in the lungs of the male BCO1−/− mice had ‘cell cycle, proliferation, oncogenes’ as their signature(Reference Piga, Van Dartel and Bunschoten326). Further inspection of the FZD6 and β-carotene controlled genes revealed a strong enrichment of genes controlled by the transcription factors JUN (the target of JNK) and activating transcription factor 2 (ATF2, a major target of MAPK14)(Reference Piga, Van Dartel and Bunschoten326). Of note, also retinoid receptors are transcription targets of JUN(Reference Altucci and Gronemeyer252). Identified JUN/ATF2 target genes included forkhead box M1 (FOXM1) as well as centromere protein (CENPE), kinesin family member 11 (KIF11) and cell division cycle associated 8 (CDCA8). FOXM1 is a transcription factor with a regulatory role in mitosis (CENPE, KIF11) and cell division (CDCA8). Strikingly, in BAES2B human lung cells, in smoke- and β-carotene-exposed ferret lungs and in β-carotene-exposed lungs of BCO1−/− mice, the JUN and MAPK14 signalling pathways are affected(Reference Wagner and Nebreda318,Reference Van Helden, Godschalk and Heil321,Reference Piga, Van Dartel and Bunschoten326) , strongly suggesting that high dosages of β-carotene can affect these developmental pathways. Does this explain carcinogenicity of β-carotene? Although it is tempting to say yes, this is not necessarily the case, since development is a normal physiological process. For example, it is well established that retinoid signalling has an important role in developmental processes, including development of the lung(Reference Maden and Hind327). Only dysregulation of developmental processes, for example, by sustained activation of JUN, can lead to aberrant cell behaviour and proliferation. Furthermore, it is not straightforward to interpret the functional consequences of JUN and MAPK14 activation, since functional effects are context dependent(Reference Wagner and Nebreda318) and depend on interaction of JUN and MAPK14(Reference Ventura, Tenbaum and Perdiguero328). Recently, it was observed in vitro in colon cancer cells that exposure to β-carotene altered miRNA expression, associated with up-regulation of histone acetylation and DNA methylation(Reference Kim, Kim and Kim329). Together with the observation in a mouse model that inactivation of BCO1, which may potentially also have affected the exposure to dietary vitamin A, affected miRNA expression associated with WNT signalling(Reference Kim, Gong and Rubin330), this hints to a role for β-carotene/vitamin A metabolism in regulating miRNAs, also affecting developmental regulatory pathways.
A possible role of the supplementation regimen exists as well. The subjects in the ATBC and CARET study were supplemented daily with a high dose of β-carotene for a long period (median of 6.1 years with 20 mg/d of β-carotene and 3.7 years with 30 mg/d of β-carotene, respectively). Such a continuous supplementation regimen differs substantially from dietary intake, which is rather variable. Continuous supplementation poses a constant pressure on associated pathways. This can possibly result in a lower threshold for dysregulation, paving the way for the increased lung cancer risk in smokers and in asbestos-exposed subjects, as was observed in the ATBC and CARET studies(Reference Omenn, Goodman and Thornquist13,Reference Albanes, Heinonen and Taylor301) . It is interesting to note that in a study that involved a less continuous exposure, with every-other-day intake of supplements, no adverse effects were observed(Reference Hennekens, Buring and Manson305). As mentioned before, this study involved non-smokers, rather than smokers, former smokers or asbestos workers as in the ATBC and CARET studies. That variable intake compared with continuous intake has a strong effect on health outcomes is exemplified by a study in APOE3L mice(Reference Wielinga, Yakala and Heeringa331). These mice are prone to developing cardiovascular disease when given a high-fat high-cholesterol diet. In this particular study, the mice were given (i) a high-fat control diet; (ii) the control diet with high cholesterol (1 %), alternating the control diet (4 d) and the control diet with high cholesterol (3 d); or (iii) the control diet with an intermediate level of cholesterol (0.43 %) corresponding to the average cholesterol intake of the alternating diet. Remarkably, the alternating diet, but not the intermediate diet, showed the strongest reduction in inflammation and atherosclerosis, with inflammation being at the level of the control diet and atherosclerosis being 50 % reduced, although still being higher than the control(Reference Wielinga, Yakala and Heeringa331). Similarly, the beneficial effects of intermittent fasting on metabolic health(Reference Antoni, Johnston and Collins332) and aging(Reference Goodrick, Ingram and Reynolds333) are clear, without inducing the strong changes in body weight and body composition that are associated with caloric restriction(Reference Speakman and Mitchell334). Dampening of signalling and induction of compensatory responses may play a role, as is suggested by the disappearance of effectiveness of anti-diabetic treatment over time(Reference Kahn, Haffner and Heise335).
Insights into possible consequences of prolonged exposure to high dosages of retinoids can be obtained from studies in which acute promeyelocytic leukaemia patients are treated with ATRA as a therapeutic agent. Without any other medication, approximately 25 % of the patients develop retinoic acid syndrome. Typical for the clinical manifestations of retinoic acid syndrome are pulmonary effects and that a large percentage of patients suffer from inflammatory infiltrates(Reference Larson and Tallman336,Reference Patatanian and Thompson337) . Although the exact mechanisms are unknown, these data indicate that an increase in retinoic acid might become detrimental under certain conditions. ATRA levels are thought to be very well regulated(Reference Loudig, Babichuk and White338). Since the measurement of ATRA involves sophisticated equipment, the ATRA precursor retinol is measured in most studies that investigated effects of carotenoids. In contrast to the assumed stable ATRA levels, consumption of β-carotene-rich carrot juice for 2 weeks resulted in stable retinol plasma concentrations but almost doubled ATRA plasma concentrations(Reference Rühl, Bub and Watzl290). Also in the studies with BCO1−/− mice, a positive correlation between β-carotene concentrations in lung and retinyl ester concentrations in the lung was observed(Reference Van Helden, Heil and Van Schooten320,Reference Van Helden, Godschalk and Heil321) . This indicates that there is a controlled balance between concentrations of β-carotene and its stored form. What happens with ATRA levels after prolonged supplementation with β-carotene is unknown, but, for example, prolonged ATRA treatment causes an increase in retinoid catabolism and can lead to retinoic acid resistance(Reference Miller339,Reference Njar, Gediya and Purushottamachar340) . This is mainly caused by an increased cytochrome P 450 family 26 (CYP26) capacity. These studies indicate that retinoid levels may be altered after β-carotene administration and that prolonged periods of elevated retinoid levels can induce an increase in retinoid catabolism. This hypothesis is more or less strengthened by some adverse effects that have been observed in ATRA therapy. Administration of ATRA can induce retinoid hyper-catabolism, and patients cannot be actively treated with ATRA after a first therapy even when the dose is doubled(Reference Muindi, Frankel and Miller341,Reference Muindi, Young and Warrell342) . Moreover, decreases in endogenous retinol stores down to 40 % have been observed in patients with exogenous retinoid therapy and resulted in adverse effects on vision(Reference Peng, Dalton and Alberts343). Moreover, retinoic acid syndrome is characterised by weight gain (ATRA increase results in the opposite(Reference Larson and Tallman336)) in association with pulmonary infiltrations. Together this suggests that prolonged exposure with β-carotene may induce changes in retinoid metabolism, possibly inducing retinoid resistance. This enforces the suggestion that the continuity of the supplementation may be a factor that could have contributed to the outcomes of the ATBC and CARET studies, although it is unclear whether this is due to retinoid resistance or prolonged activation of frizzled signalling or both.
Genotypes and susceptibility to develop lung cancer following β-carotene supplementation
Although it is acknowledged that high doses of β-carotene can increase the risk to develop lung cancer in smokers or subjects who have been exposed to asbestos, it is important to note that only a low percentage of the subjects who took β-carotene supplements in the ATBC and CARET studies developed lung cancer. More precisely, 72 subjects out of 7283 of the ATBC study and 49 subjects out of 9420 subjects of the CARET study developed lung cancer, which was apparently due to the β-carotene supplement. This shows that only a very small fraction of the population, i.e. about 0.5–1 % of the subjects who smoked and/or had been exposed to asbestos were sensitive to the β-carotene supplement. The most likely explanation of this observation is that these subjects bore genotypes that led to the adverse effect of β-carotene. Indeed, most of the phenotypic differences between individuals, as well as their susceptibility to develop different diseases, are due to genetic variations(Reference Altshuler, Gibbs and Peltonen344,Reference Auton, Abecasis and Altshuler345) . We hypothesise that different genotype(s) can be responsible for the adverse effect of β-carotene on lung cancer. These genotypes can be located in the genes that are implicated in either the bioavailability or the metabolism of β-carotene or in the interaction between β-carotene or, most likely, its metabolites and the genes involved, directly or indirectly, in lung cancer. Indeed, we believe that genotypes that modulate the bioavailability and the metabolism of β-carotene can modify both the concentration of the parent molecule and its metabolites in the sites where they modulate the mechanisms involved in the development of lung cancer. Although all these genetic variations are far from known, some of them have been identified and have been listed in recent reviews(Reference Borel and Desmarchelier307,Reference Borel346–Reference Desmarchelier, Landrier and Borel348) . The second set of genes where some genetic variations might explain the sensitivity of some subjects to develop lung cancer following β-carotene supplementation is the four genes that have been suggested to be involved in the adverse effect of β-carotene on lung cancer, i.e. ADH7, JNK, MAPK14 and JUN. Of course, these genes exhibit genetic polymorphisms (https://www.ncbi.nlm.nih.gov/snp), and several studies have shown association between genetic variants at these genes and cancer. It is not the topic of this review to comprehensively list all these associations; thus, only some illustrating references are provided. For example, a genetic variant in ADH7 (rs1573496) has been associated with aerodigestive cancer(Reference Hashibe, McKay and Curado349). Furthermore, carriers of MAP2K7 rs3679T genetic variant had an increased risk of lung cancer(Reference Jia, Zhu and Zhou350). Finally, some genetic variants of C-Jun increase the risk of lung cancer via interaction with smoking or drinking(Reference Huang, Liu and Yang351).
In summary, it is highly likely that the adverse effects of β-carotene that were observed in about 1 % of the subjects who were enrolled in the ATBC and CARET studies were due to peculiar genotypes in the genes involved in either the bioavailability and the metabolism of β-carotene and/or in lung cancer related genes that are targets for β-carotene metabolites. Future studies additionally genotyping their volunteers will perhaps confirm this hypothesis and identify genotypes, or more likely combinations of genotypes, which result in an increased risk to develop a lung cancer when taking additional β-carotene supplements.
Health-beneficial effects of carotenoids and carotenoid degradation products. More matters?
Due to the limited knowledge in the area of carotenoid metabolites, functioning as ligands of nuclear hormone ligands and further signalling, it should be discussed whether more ligands and more nuclear hormone mediated signalling is purely seen as beneficial or might also be seen as detrimental for human health. RAR-mediated signalling is responsible for a large variety of physiologically mediated pathways, ranging from central physiological pathways such as the induction of differentiation(Reference Janesick, Wu and Blumberg352,Reference Mark, Teletin and Vernet353) , apoptosis(Reference Jiménez-Lara, Clarke and Altucci354,Reference Szondy, Reichert and Fésüs355) , cell cycle control(Reference Dimberg and Öberg356–Reference Sacha, Zawada and Dulińska-Litewka358), lipid homeostasis(Reference Bonet, Ribot and Palou187,Reference He, Gong and Fang359,Reference Rühl and Landrier360) and proliferation(Reference Janesick, Wu and Blumberg352,Reference Mosher and Schaffer361) , which are of high importance for processes of embryonic development(Reference Lammer, Chen and Hoar362,Reference Ghyselinck and Duester363) , reproduction(Reference Li, Long and Xie364,Reference Clagett-Dame and Knutson365) , epidermal homeostasis and regeneration(Reference Ghyselinck, Chapellier and Calleja366,Reference Chapellier, Mark and Messaddeq367) , immune responses(Reference Erkelens and Mebius368,Reference Rühl369) and maintenance of bone, brain, nervous and cardiovascular functions(Reference Rhinn and Dollé370–Reference Krezel, Ghyselinck and Samad372). Too low or too high levels of endogenous ATRA(Reference Lee, Leung and Tang373), due to a dysfunctional or stressed retinoid homeostasis, while hard to define what the upper and lower threshold levels are, were seen to be risk factors for various previously mentioned physiological functions and are associated with further disease development, where retinoid signalling is dysfunctional. It is commonly accepted that too low retinoid levels are associated with cancer, obesity, reduced immune responses and disorders of the nervous system. Provitamin A carotenoids, as major retinoid precursors, can therefore be seen as dietary derivatives with a unique beneficial function, especially for these listed dysfunctions related to retinoid signalling. Alternatively, too high levels of retinoid-mediated signalling were also associated with various diseases in these specific areas, including diabetes(Reference Erkelens and Mebius368,Reference Blaner374,Reference Landrier, Kasiri and Karkeni375,Reference Petrosino, Disilvestro and Ziouzenkova376) , increased immune responses/allergies(Reference Mihály, Gericke and Lucas377–Reference Stephensen, Borowsky and Lloyd381) and osteoporosis(Reference Henning, Conaway and Lerner382).
Besides functioning as a precursor for the RARs, carotenoids were also reported to be precursors of RXR ligands(Reference Krężel, Rühl and de Lera107,Reference Shaish, Harari and Hananshvili383) . RXR-mediated signalling includes the controversially discussed RXR-RXR-mediated signalling while mainly functioning as a heterodimer binding partner for permissive and non-permissive heterodimers(Reference Desvergne384,Reference Szanto, Narkar and Shen385) . Permissive signalling is mainly mediated via RXR-PPAR- and RXR-LXR-mediated signalling(Reference Mangelsdorf and Evans131). Especially lipid metabolism and homeostasis are major axes under control of these pathways(Reference Desvergne384,Reference Evans and Mangelsdorf386) and seen as highly beneficial for the prevention of cardiovascular diseases(Reference Lenhard387,Reference Shulman and Mangelsdorf388) . We predict that certain carotenoids might be preferred nutritional precursors of RXR ligands(Reference Rühl, Krezel and de Lera106) and therefore might be highly beneficial for the prevention of cardiovascular diseases.
Besides the regular recommendation of ‘more matters’, referring to dietary intake of carotenoids, preferably distinct single and multiple carotenoid dietary suggestions should be focused on. This is especially relevant for carotenoids such as lutein and zeaxanthin, which are, in contrast to β-carotene, lycopene and β-cryptoxanthin, not predominantly consumed via natural food, from food with added ingredients or even targeted dietary supplements. Their intakes are determined exclusively by fruit and vegetable intake, which is low to intermediate in large groups of Western society. More targeted specific recommendations for selected groups of fruits and vegetables or, alternatively, nutritional extracts high in specific carotenoids or even dietary supplements may further boost a specific carotenoid intake and adaptations for specific populations, such as for certain age and gender groups, lifestyles, ethnicities, athletes, etc., may be envisioned.
In addition, as summarised, we observe a large variety in endogenous levels of carotenoids in humans, which not only depends on individual dietary intake but is mainly due to our human genetic diversity (reviewed in ref.(Reference Bohn, Desmarchelier and Dragsted168)). Here, a focus should be put on further examination of carotenoid metabolite levels, nuclear hormone mediated ligand signalling monitoring with transcriptomic or other omics techniques to ensure a healthy life. Further approaches, for instance, personal nutrition based on individual needs, should be also highlighted for nutrition-related carotenoid research.
Conclusions and dietary suggestions
Carotenoids may act in humans as antioxidants and photo-protective compounds, while likely a larger activity range is mediated via the transcription factors NF-κB and NRF2 as well as the nuclear hormone receptors RARs and RXRs. It is likely that the list of transcription factors or nuclear receptors activated by carotenoids and more specifically by a larger variety of identified and even not yet identified carotenoid metabolites is far from being conclusively identified.
With relevance for larger population groups, specific epigenetic mechanisms are co-involved in carotenoid-mediated signalling. This new field of research will require intensive investigations regarding the influence starting from carotenoid intake, towards carotenoid and further carotenoid-metabolite levels and transmitted biological signalling.
In addition, as carotenoids are well-known markers of fruit and vegetable intake, their blood levels in humans may simply function as indicators of food intake, lifestyle and health markers.
Based on this review, the following dietary suggestions can be drawn:
-
A. A high intake of fruits and vegetables that are rich in a variety of carotenoids, and many other beneficial compounds, is advised. An upper limit for fruit and vegetable intake is not needed, because it seems unlikely that even the highest intake of fruit and vegetables is likely to pose any adverse risk from the carotenoid point of view.
-
B. Single carotenoids as supplements, except for lutein and zeaxanthin for eye health, did until now not conclusively show any health beneficial effects. Possibly, a more complex dietary supplementation and combination may be needed to yield clear health-beneficial effects, which may depend on each individual’s personal needs. High-dose supplement intake of β-carotene is warned against by the EFSA for individuals at risk for lung cancer, i.e. (previous) smokers (Reference Younes, Aquilina and Castle389) .
-
C. Simple recommendations using terms such as ‘more matters’ and ‘antioxidants are good’ may do more harm than good and are detrimental for a clear evaluation of beneficial health effects of carotenoids and impairs advice for a healthy balanced diet and/or targeted carotenoid supplementation.
Acknowledgements
The concept of the publication and its final layout was created thanks to the involvement of J.D-L, R.R and T.B. Contribution in the preparation of chapters and tables, in writing publications: M.L.B.; P.B.; J.C-R.; Y.v H.; J.K.; J-F.L.; M.R.L.; B.O.A.; B.P.; J.R.; M. P.; P.R.; J.R.; B-W.R.; Y.S.; P.T.; R.T.; A.W.; R.R.; J.D-L. All authors read and agreed on the final version.
Financial support
This article is based on work from the European Cooperation in Science and Technology (COST) Action CA15136, European Network to Advance Carotenoid Research and Applications in Agro-Food and Health (www.eurocaroten.eu), supported by COST.
Conflicts of interest
The authors declare no conflicts of interest.
Individual author contribution
The entire manuscript, summary of sections and conclusions/dietary suggestions were set up and directed by the three equal contributors Dr. Joanna Dulińska-Litewka, Dr. Torsten Bohn and Dr. Ralph Rühl.
The introduction was written by Torsten Bohn. Each chapter was mainly compiled by individual groups of co-authors: Sub-chapter ‘Antioxidant activities’ by Dr. Irina Milisav. Sub-chapter ‘Activation of antioxidant/anti-inflammatory cascades involving the transcription factors NF-κB and NRF2’ by Drs. Yoav Sharoni and Jean-Francois Landrier. Part about Carotenoid metabolites and retinoids as activators of nuclear hormone receptors’ by Ralph Rühl and Joanna Dulińska-Litewka. Sub-chapter ‘Bioavailability of carotenoids and alternative mechanisms of carotenoids’ by Torsten Bohn. Sub-chapter ‘Disease-related mechanisms potentially modulated via carotenoid-gut microbiota interaction’ by Marisa Porrini, Patrizia Riso and Torsten Bohn. Sub-chapter ‘Carotenoids and obesity’ by Drs. Joan Ribot, M. Luisa Bonet and Jean-Francois Landrier, Sub-chapter ‘Carotenoids and cancer’ by Drs. Patrick Borel, Ralph Rühl, Jaap Keijer, Joanna Dulińska-Litewka, Yvonne van Helden. Chapter ‘Health-beneficial effects of carotenoids and carotenoid degradation products. More matters?’ by Ralph Rühl.






