Introduction
Dietary fat has long been implicated in the aetiology of CVD. The majority of research into the role of dietary fat in CVD has focused on the effects of dietary fats on lipoprotein metabolism, due to the well-characterised association between blood cholesterol levels and cardiovascular mortality(Reference Neaton and Wentworth1). The strength of the evidence enabled the prediction of changes in blood cholesterol, LDL-cholesterol, HDL-cholesterol and TAG that would occur on diets varying in their total fat content and fatty acid composition in a meta-analysis of sixty studies(Reference Mensink, Zock and Kester2). Clearly the field of nutritional science has made great inroads into our understanding of how dietary fats may affect cardiovascular risk through their effects on lipoprotein metabolism. Recommendations for dietary fat intake by the UK government are based on this evidence; it appears that the average British diet is edging closer to the dietary fat guidelines but there is still a need for a reduction in population intake of SFA, and an increase in some unsaturated fats (Table 1). In addition to lipoprotein metabolism, dietary fats may also exert effects on less well-researched components of cardiovascular risk such as insulin sensitivity, haemostasis or vascular function. The purpose of the present review is to offer a new perspective on the role that the amount of dietary fat, as well as the fatty acid composition (SFA, PUFA and MUFA), may have in the maintenance of normal blood vessel tone.
Table 1 UK dietary reference values for fat in adults
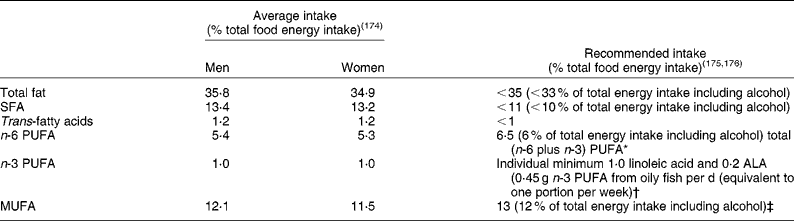
* Recommended population average intake for total PUFA is 6·5 % of total food energy, with an individual maximum intake of 10 % energy intake, and an n-6:n-3 PUFA ratio of 5:1.
† Only 27 % of the UK population consumes oily fish, so the majority of the population will have intakes below the population average.
‡ If other guidance is followed, average population intake should be 13 % of total food energy.
Vascular function and cardiovascular risk factors
The ability of the vascular tree to respond and adapt to the demands placed upon it is critical to the lifelong development of atherosclerosis and eventual CVD. Vascular function is a general term used to describe the regulation of blood flow, arterial pressure, capillary recruitment and filtration and central venous pressure, all of which are controlled by a multitude of intrinsic mechanisms (for example, NO, prostacyclin (PGI), adenosine, histamine, the stretch-activated Bayliss myogenic response, etc) and extrinsic mechanisms (sympathetic and parasympathetic innervation, adrenaline, angiotensin, vasopressin and insulin). Components of vascular function, such as hypertension, arterial stiffness and endothelium-dependent vasodilation, are associated with cardiovascular mortality(Reference Kannel3–Reference Yeboah, Crouse and Hsu5), and are therefore important risk factors that may be targeted with dietary modification.
Endothelial function measurements
The function of conduit (muscular) and terminal (resistance) arteries can be assessed by methods designed to measure vasodilation and vasoconstriction, mainly determined by endothelial mechanisms. Endothelial dysfunction (comprising increased permeability, reduced vasodilation, and activation of thrombotic and inflammatory pathways) is a crucial factor in the early stages of atherosclerosis(Reference Ross6). Prolonged activation of vascular mechanisms for protecting against adverse stimuli (inflammatory response, procoagulation and vasoconstriction) can lead to endothelial dysfunction. Endothelium-dependent vasodilation is mainly mediated by NO, which is released from the endothelium following activation of the enzyme endothelial NO synthase (eNOS), causing the underlying smooth muscle to relax(Reference Triggle, Hollenberg and Anderson7). Other endothelium-dependent vasodilatory factors include PGI and endothelium-derived hyperpolarising factor(Reference Triggle, Hollenberg and Anderson7). The endothelium also secretes vasoconstricting factors, the major vasoconstrictor being the peptide endothelin-1. Figure 1 illustrates some of the endothelium-dependent mechanisms that are known to mediate arterial vasodilation and vasoconstriction.

Fig. 1 Outline of major mechanisms for vascular smooth muscle cell relaxation and contraction mediated by the endothelial cell. Shear stress arising from blood flow increases intracellular Ca2+ levels. A rise in endothelial Ca2+ triggers the production of three relaxing factors: NO, prostacyclin (PGI2) and endothelium-derived hyperpolarising factor (EDHF) which diffuse to the smooth muscle cell leading to relaxation. Increased intracellular Ca2+ activates endothelial NO synthase (eNOS), which then converts l-arginine (l-Arg) to l-citrulline, and NO is released. NO diffuses to the vascular smooth muscle cell where it activates soluble guanylate cyclise (sGC), causing an increase in cGMP production and consequently a decrease in smooth muscle intracellular Ca2+ and relaxation. PGI2 interacts with the PGI2 receptor, elevating cAMP levels and decreasing intracellular Ca2+, leading to relaxation of the smooth muscle. EDHF is a vasorelaxant that has not been definitively identified and which can cause vasorelaxation by hyperpolarising vascular smooth muscle cells. Stimulation of endothelin-1 (ET-1) production by stress stimuli occurs in the endothelial cell. ET-1 binds to endothelin receptor A (ETA) and endothelin receptor B (ETB) receptors on the vascular smooth muscle cell, activating the phosphatidylinositol 4,5-bisphosphate–inositol 1,4,5-trisphosphate (PIP–IP3) pathway and triggering contraction by increasing intracellular Ca2+ levels. AA, arachidonic acid; COX, cyclo-oxygenase; PI3K, phosphoinositide-3 kinase; PGH2, PG H2; PGIS, prostacyclin synthase; Gq, Gq protein; AC, adenylyl cyclase.
Flow-mediated dilatation (FMD) of the brachial artery is now regarded as the most reliable assessment of endothelium-dependent vasodilation and as a surrogate measure of NO production(Reference Moens, Goovaerts and Claeys8). It uses ultrasound to record images of the endothelium-dependent dilatory responses of the brachial artery caused by reactive hyperaemia (FMD)(Reference Celermajer, Sorensen and Gooch9). Another method involves venous occlusion plethysmography to measure forearm blood flow in peripheral resistance vessels following infusion of acetylcholine(Reference Fichtlscherer, Rosenberger and Walter10). A third method uses laser Doppler imaging to measure peripheral microvascular endothelial function, a technique that assesses the response of cutaneous blood vessels to transdermal delivery of endothelium-dependent (for example, acetylcholine) and endothelium-independent (for example, sodium nitroprusside) vasoactive agents by iontophoresis(Reference Morris and Shore11). These methods have been widely adopted and shown to be reasonable prognostic indicators of cardiovascular events in patients with vascular diseases(Reference Gokce, Keaney and Hunter12–Reference Heitzer, Schlinzig and Krohn16).
Arterial stiffness and compliance measurements
Arterial stiffness (the inverse of arterial compliance) is mainly a consequence of changes in arterial wall composition in the systemic elastic arteries (loss of integrity of the elastin and increased collagen formation) and therefore can be quantified as a measure of ageing, hypertension and development of arteriosclerosis in the large central elastic arteries(Reference Nichols17). Muscular arteries are unaffected by these kinds of age- and hypertension-related changes, and drugs that are designed to reduce blood pressure by vasodilation have only indirect effects on the central elastic arteries via alteration of wave reflection amplitude and timing(Reference Kelly, Millasseau and Ritter18, Reference McEniery, Wallace and Mackenzie19). However, endothelial dysfunction of the conduit and terminal arteries may exacerbate arterial stiffness by increasing peripheral resistance due to an imbalance in vasodilators and vasoconstrictors(Reference Nichols17). Measurements of arterial compliance are commonly used as an indicator of arterial stiffening or ageing, but they are also indicative of endothelial function to a limited extent, since peripheral arterial compliance is partly dependent on endothelium-dependent vasodilation(Reference Kelly, Millasseau and Ritter18). Information about the elasticity of arteries and peripheral vasodilation can be gleaned from arterial pulse wave analysis. Following ventricular ejection, the pulse pressure wave begins in the aorta. Stroke volume and the elasticity of the central aorta determine the peak pressure of the initial pulse wave during systole. Aortic compliance (the ability of the walls of the aorta to expand to accommodate the increase in blood volume) is therefore directly related to pulse pressure. As the pulse pressure wave travels beyond the aorta, through smaller conduit and muscular arteries, and then arterioles, a second reflected pressure wave occurs as the blood flow passes bifurcations in the arterial tree and encounters increased resistance(Reference Levick20).
Pulse wave velocity (PWV) is a common method for assessing arterial stiffness(Reference Mattace-Raso, van der Cammen and Hofman4), is associated with all-cause mortality and cardiovascular outcomes(Reference Ter Avest, Stalenhoef and de Graaf21) and is regarded as a ‘gold standard’ measurement(Reference Laurent, Cockcroft and Van Bortel22). PWV involves applanation tonometry (alternatively MRI or Doppler ultrasound) to measure the pressure wave of the carotid and femoral arteries commonly, although other sites can be used. PWV is usually measured as the delay between the initial upstrokes of the initial pulse pressure peak at the carotid and femoral pulse sites (adjusted for anatomical distance)(Reference Nichols17). The smaller the delay between the corresponding points on the upstroke of the initial wave (therefore the higher the velocity) the stiffer the arteries. An analogous measure of arterial stiffness, requiring minimal training of the observer, can be produced using the digital volume pulse (DVP) method, whereby finger photoplethysmography yields the digital pulse pressure waveform in order to calculate a stiffness index(Reference Millasseau, Kelly and Ritter23). Further methodological techniques and considerations in the measurement of arterial stiffness are reviewed in detail elsewhere(Reference Nichols17, Reference Ter Avest, Stalenhoef and de Graaf21, Reference Laurent, Cockcroft and Van Bortel22, Reference Hamilton, Lockhart and Quinn24). The radial or carotid artery pressure waves can also be used to calculate the augmentation index: the ratio of the magnitude of the reflected wave to the initial wave(Reference Millasseau, Patel and Redwood25). This is an indirect measure of arterial stiffness and is mainly determined by the timing of return of the reflected wave, since an earlier return will increase the amplitude of the reflected wave by occurring in systole rather than diastole. However, it is also affected by changes in vascular tone in the muscular arteries (itself determined by release of vasodilators such as NO and vasoconstrictors such as endothelin-1), and can vary independently of PWV(Reference Kelly, Millasseau and Ritter18). The equivalent measure using the DVP is the reflection index and is thought to represent changes in vascular tone in the periphery(Reference Millasseau, Kelly and Ritter26).
Blood pressure measurements
Although changes in arterial stiffening are the most accurate method to monitor the effects of ageing, systolic and diastolic blood pressure measurements have been a valuable routine tool for many years in the monitoring of vascular changes and prediction of cardiovascular risk. Mean arterial pressure is determined by cardiac output and total peripheral resistance, and systolic and diastolic blood pressures depend on the size of the oscillation in pressure either side of mean arterial pressure(Reference Levick20). Systolic blood pressure is the peak arterial pressure during systole and depends on peripheral wave reflection, arterial stiffness and stroke volume, whereas diastolic blood pressure is the lowest pressure during cardiac relaxation and is primarily influenced by the tone of small arteries and arterioles(Reference Levick20). Many other factors regulate blood pressure, including the sympathetic nervous system and the kidneys, but since hypertension leads to vascular dysfunction, as well as being a function of arterial tone, it is a key parameter for vascular function. The latest British Hypertension Society classification of blood pressure levels is given in Table 2(Reference Williams, Poulter and Brown27). Blood pressure measurements were traditionally carried out using a mercury sphygmomanometer, but they are now usually carried out with a semi-automatic oscillometric electronic blood pressure monitor for clinic and home measurements. Ambulatory blood pressure measurement has been shown to be a good predictor of cardiovascular risk and avoids a number of sources of error that occur with clinic measurements(Reference Pickering, Shimbo and Haas28). In particular, ambulatory blood pressure monitoring gives a more accurate overview of blood pressure since there are many repeat measurements and they are taken over a range of different activities, giving a true picture of average 24 h, day-time and night-time blood pressure. Furthermore, ambulatory blood pressure monitoring reduces the risk of artificial values due to influences such as white-coat hypertension(Reference Pickering, Shimbo and Haas28). Slightly different classifications are adopted when interpreting data from ambulatory blood pressure monitors (Table 2).
Table 2 Guidelines for classification of clinic and ambulatory blood pressure levels (mmHg)*

* If clinic systolic blood pressure and diastolic blood pressure fall into different categories, the higher value should be taken for classification. No classification is given to differentiate between mild, moderate and severe hypertension using ambulatory blood pressure monitoring due to lack of evidence to base recommendations upon.
Dietary fat and vascular function
Although it is generally accepted that salt, alcohol and fruit and vegetable intakes can modulate blood pressure, the effects of dietary fatty acids on vascular function are less well characterised in the literature. The remainder of the present review will evaluate the nature and the strength of evidence for the chronic and acute influence of total fat on blood pressure, arterial compliance and endothelial function, and will then consider the differential effects of saturated and unsaturated fatty acids from observational and both chronic and acute intervention studies. The n-3 long-chain PUFA (LCP) EPA and DHA have been investigated more extensively and appear to have distinct mechanisms of action upon the vasculature, and therefore the evidence for EPA- and DHA-modulated effects on arterial function will be examined in a separate section.
Total fat
Epidemiological and chronic intervention studies
Total fat as a proportion of energy intake does not seem to have a strong effect on the risk of CHD when other dietary variables are adjusted for, according to the results of the Nurses' Health Study, which reported incidence of CHD in 80 082 women 14 and 20 years after baseline assessment(Reference Hu, Stampfer and Manson29, Reference Oh, Hu and Manson30). In fact, a number of studies showed that blood pressure may actually be reduced by a high-fat diet (specifically high-MUFA) compared with a high-carbohydrate diet, in populations at risk of CVD(Reference Appel, Sacks and Carey31–Reference Shah, Adams-Huet and Bantle33). Evidence showing low-fat diets to be beneficial for the regulation of blood pressure may reflect the reduction in SFA rather than total dietary fat per se (Table 3). Two diets with similar MUFA content, a high-fruit-and-vegetable/high-fat diet, and a high-fruit-and-vegetable/low-fat diet, were compared with a control diet using ambulatory blood pressure monitoring in the Dietary Approaches to Stop Hypertension (DASH) trial(Reference Moore, Vollmer and Appel34). Both diets reduced blood pressure but the low-fat diet had a greater effect, possibly due to reduced SFA content, although other dietary components such as increased intake of wholegrain foods and Ca may have also played a part. The overall picture for high-fat v. low-fat diets affecting blood pressure is muddled by the failure to keep the type of fat constant, and the few studies that have attempted to control for fatty acid composition report no differences(Reference Brussaard, van Raaij and Stasse-Wolthuis35–Reference Mensink, Janssen and Katan37). Few studies have been conducted with regards to other measures of vascular function. Cross-sectional analysis of a cohort of children aged 10 years suggested that total fat intake was associated with arterial stiffness, independently of dietary fatty acid composition, suggesting that the total amount of fat in the diet may influence arterial integrity from an early stage in life(Reference Schack-Nielsen, Molgaard and Larsen38). However, high-fat v. low-fat dietary intervention studies (also differing in type of fat) had no differential effect on arterial stiffness or endothelial function(Reference Ashton, Pomeroy and Foster39–Reference Keogh, Brinkworth and Noakes41). Overall, the evidence suggests that total fat per se does not have a strong effect on vascular function and that dietary fatty acid composition may have a more important bearing.
Table 3 Dietary intervention studies on chronic and acute effects of total fat intake on blood pressure (BP) or vascular function

PAL, parallel design; CO, cross-over design; M, men; W, women; H-PUFA, high-PUFA; LF, low-fat; L-PUFA, low-PUFA; HF, high-fat; H-SFA, high-SFA; H-MUFA, high-MUFA; T2D, type 2 diabetics; H-CHO, high in carbohydrate; ABP, ambulatory blood pressure; L-GI, low glycaemic index; H-F&V, high in fruit and vegetables; VLF, very low-fat; HT, hypertensive; PWV, pulse wave velocity; FMD, flow-mediated dilatation; DVP, digital volume pulse; DVP-SI, stiffness index measured by the DVP; MF, medium fat; FBF, forearm blood flow; EDV, endothelium-dependent vasodilation.
* Source: olive oil.
† Single-meal (uncontrolled) studies excluded.
‡ 300 kcal = 1255 kJ; 342 kcal = 1431 kJ; 478 kcal = 2000 kJ.
Acute intervention studies
Studies of postprandial responses to dietary fat are more straightforward and less time-consuming to conduct compared with chronic dietary intervention studies. Furthermore, single-meal interventions are not beset by problems such as non-compliance to dietary advice. Possibly for these reasons, there are now at least fifteen studies that have investigated the acute effects of high-fat meals(Reference Bae, Schwemmer and Lee42–Reference Fard, Tuck and Donis56), and at least eight studies reporting the relative acute effects of saturated and unsaturated fat on endothelial function(Reference Berry, Tucker and Banerji57–Reference Vogel, Corretti and Plotnick63). These studies provide information about the stress imposed on the endothelium by everyday postprandial exposure to increased TAG and NEFA, and allow us to discover the optimum fatty acid composition of a meal in order to reduce any adverse effects on vascular function, ultimately protecting against long-term arterial damage.
In general, high-fat meals have been shown to impair endothelium-dependent vasodilation, mostly using FMD methodology(Reference Bae, Schwemmer and Lee42, Reference Ong, Dean and Hayward44, Reference Padilla, Harris and Fly45, Reference Tsai, Li and Lin49–Reference Westphal, Taneva and Kastner51, Reference Vogel, Corretti and Plotnick54–Reference Fard, Tuck and Donis56, Reference Ling, Zhao and Gao64), but also forearm blood flow(Reference Shimabukuro, Chinen and Higa46, Reference Steer, Sarabi and Karlstrom48). Furthermore, high-fat meals may impair systemic arterial compliance(Reference Nestel, Shige and Pomeroy65). In contrast to these findings, Djousse et al. (Reference Djousse, Ellison and McLennan43) did not report impaired FMD after a meal of burger and chips, possibly due to the added effect of the protein in the meal, which has been shown to prevent dietary fat-induced endothelial dysfunction(Reference Westphal, Taneva and Kastner51). An Australian group, using venous occlusion strain-gauge plethysmography to measure forearm blood flow(Reference Skilton, Lai and Griffiths47, Reference Raitakari, Lai and Griffiths61), stand alone in reporting increased endothelium-dependent vasodilation following a high-fat meal, possibly due to methodological differences. Interestingly, Skilton et al. showed that the increase in forearm blood flow after a high-fat meal (61 g fat, providing 53 % of total energy) was diminished with age, but unaffected by insulin sensitivity(Reference Skilton, Lai and Griffiths47). Further confusion is added by the fact that Williams et al. demonstrated impaired FMD after a high-fat meal (64 g fat) using deep-fried cooking oil (obtained from a restaurant), but not a high-fat meal using unheated cooking oil(Reference Williams, Sutherland and McCormick52), but in a second paper they reported no impairment in FMD following 78 g of either heated (used to deep-fry potato chips) or unheated safflower-seed and olive oils(Reference Williams, Sutherland and McCormick53). Rueda-Clausen et al. compared three types of oils at two levels of deep-frying and showed that all the heated and unheated fats induced a reduction in FMD of approximately 32 %(Reference Rueda-Clausen, Silva and Lindarte62). Clearly there is little evidence so far that deep-frying oil can acutely affect endothelial function over and above the effect of the total amount of fat, although the longer-term effects of habitual consumption on arterial health are unknown.
Dietary saturated v. unsaturated fatty acids
Altering the fatty acid composition of the diet necessarily involves the manipulation of more than one component. For example, reduction of SFA in the diet will require replacement with another type of fatty acid or another macronutrient in order to avoid the confounding effect of energy deficits. Therefore, it is more useful to consider the influence of the dietary fatty acid profile as a whole (SFA, MUFA and PUFA) rather than each type of fatty acid in isolation. Since n-3 LCP have been studied more extensively and may have distinct mechanisms of action, current knowledge on their vascular effects will be examined in a separate section.
Cross-sectional studies
Table 4 summarises a selection of cross-sectional studies that have investigated potential associations between dietary fatty acid intake (estimated from dietary assessment methods or by using biomarkers of intake) and vascular function. Cross-sectional studies assessing dietary intake via food records(Reference Williams, Fortmann and Terry66, Reference Salonen, Salonen and Ihanainen67) have shown that PUFA intake is inversely related to blood pressure whereas SFA intake is positively related to blood pressure, with some studies finding no relationship(Reference Dauchet, Kesse-Guyot and Czernichow68). Dietary fat intake as assessed by 24 h recall or dietary records from relatively small sample sizes probably do not provide reliable estimates of associations with vascular function due to well-documented methodological constraints(Reference Bingham69). However, since dietary fatty acid intake is a major determinant of tissue fatty acid profile, serum, plasma, erythrocyte or adipose tissue fatty acid composition data can be used as biomarkers of dietary fatty acid intake. The strength of the relationship between dietary and tissue fatty acid composition depends on the tissue type, and indeed the fatty acid type. Fatty acids that are synthesised endogenously and that make up a large proportion of the tissue fat, such as oleic acid and SFA, do not show close associations between dietary intake and tissue composition. However, fatty acids that are not synthesised in the body and are not present in large amounts in the diet, such as n-3 PUFA, trans-fatty acids and linoleic acid (LA) make for better predictors when measured in plasma or tissue(Reference Hodson, Skeaff and Fielding70). Serum cholesteryl ester fatty acid data from the Multiple Risk Factor Intervention Trial (MRFIT) study(Reference Simon, Fong and Bernert71), plasma phospholipid fatty acid data from the Nordland Health study(Reference Grimsgaard, Bonaa and Hansen72), and adipose tissue fatty acid data(Reference Oster, Arab and Schellenberg73–Reference Riemersma, Wood and Butler75) all yielded fatty acid-specific associations with blood pressure in men, although there were no associations with erythrocyte fatty acids in women(Reference Ciocca, Arca and Montali76). In the MRFIT study, cholesteryl ester stearic acid (18 : 0) was higher when blood pressure was lower but palmitic acid (16 : 0) was not related(Reference Simon, Fong and Bernert71), unlike the majority of studies that showed that palmitic acid was positively associated with blood pressure(Reference Grimsgaard, Bonaa and Hansen72, Reference Rubba, Mancini and Fidanza74, Reference Zheng, Folsom and Ma77, Reference Miettinen, Naukkarinen and Huttunen78). Oster et al. demonstrated an inverse relationship between adipose tissue LA (18 : 2n-6) and blood pressure(Reference Oster, Arab and Schellenberg73), which agreed with subsequent serum phospholipid data in a subgroup of a cohort of middle-aged men(Reference Miettinen, Naukkarinen and Huttunen78), but not adipose tissue analysis in middle-aged American men where it was shown that α-linolenic acid (ALA; 18 : 3n-3), not LA, was inversely associated with blood pressure(Reference Berry and Hirsch79). The Paris Prospective Study 2 found that palmitoleic acid (16 : 1n-7) was the only fatty acid related to blood pressure in men; this positive relationship was only apparent in alcohol drinkers(Reference Cambien, Warnet and Vernier80). Overall, the evidence for associations between biomarkers of dietary fat intake and blood pressure is confusing, and the lack of consistency may reflect the variety of tissues and subfractions of serum or plasma used. Recently a large cross-sectional study (INTERnational collaborative of MAcronutrients and blood Pressure; INTERMAP) attempted to clarify the relationship with LA intake by using four × twenty-four dietary recalls and eight × clinic blood pressure measurements over 3 weeks in seventeen populations in Japan, China, UK and USA (n 4680)(Reference Miura, Stamler and Nakagawa81). Multiple regression analyses showed there was an inverse relationship between dietary LA intake and blood pressure, which was strongest in the subgroup that was not receiving any prescribed or non-prescribed nutritional or medical intervention and had no history of CVD or diabetes.
Table 4 Cross-sectional studies on dietary saturated and unsaturated fatty acids (FA) intake and blood pressure (BP) and vascular function

M, men; LA, linoleic acid; PA, palmitic acid; AA, arachidonic acid; ALA, α-linolenic acid; W, women; POA, palmitoleic acid; SA, stearic acid; ETA, eicosatrienoic acid; DGLA, dihomo-γ-linolenic acid; OA, oleic acid; GLA, γ-linolenic acid; FBF, forearm blood flow; EFI, endothelial function index; EDV, endothelium-dependent vasodilation; EIDV, endothelium-independent vasodilation; PWV, pulse wave velocity.
Cross-sectional analyses using other markers of vascular function demonstrate a potential relationship between serum fatty acid levels and endothelial function(Reference Lind, Sodergren and Gustafsson82–Reference Steer, Vessby and Lind84). Both palmitic acid (16 : 0) and palmitoleic acid (in cholesteryl esters and phospholipids) were inversely associated with an index of endothelial function derived from forearm blood flow measurements (ratio of endothelium-dependent vasodilation to endothelium-independent vasodilation) in predominantly middle-aged men and women, whereas phospholipid oleic acid (18 : 1n-9) and cholesteryl ester LA were positively associated(Reference Sarabi, Vessby and Millgard83). The same study showed that phospholipid ALA was positively associated with both endothelium-dependent and endothelium-independent vasodilation, suggesting that the protective effects of this fatty acid were exerted through different mechanisms to those of oleic acid and LA(Reference Sarabi, Vessby and Millgard83). Some of these relationships were also present in younger men (SFA inversely associated with endothelial function index, and ALA positively associated with endothelium-dependent vasodilation), but not in younger women(Reference Steer, Vessby and Lind84). Despite the influence of total dietary fat intake on arterial stiffness (PWV) in children, no associations were found with dietary fatty acid composition(Reference Schack-Nielsen, Molgaard and Larsen38).
Longitudinal studies
The Nurses' Health Study, a prospective cohort study carried out in over 80 000 women, showed that there is a greater risk of CHD with increasing intake of SFA, and that PUFA, and to a lesser extent MUFA, are protective(Reference Hu, Stampfer and Manson29). There are few of these types of prospective epidemiological studies that have investigated the incidence of hypertension and none to the author's knowledge on arterial stiffness or endothelial dysfunction. Studies that have estimated dietary fat intakes at baseline and related to blood pressure at follow-up have produced mixed results(Reference Dauchet, Kesse-Guyot and Czernichow68, Reference Ascherio, Rimm and Giovannucci85, Reference Stamler, Caggiula and Grandits86). There were no associations between total fat, SFA or PUFA intake at baseline and risk of hypertension during 4 years of follow-up(Reference Ascherio, Rimm and Giovannucci85). In agreement with this, no associations were shown between intake of dairy products or Key's score at baseline with change in blood pressure at follow-up (median 5·4 years) in the Supplémentation en Vitamines et Minéraux Antioxydants (SU.VI.MAX) study(Reference Dauchet, Kesse-Guyot and Czernichow68). However, the Multiple Risk Factor Intervention Trial (MRFIT) study group reported that the average dietary intake of PUFA over 6 years, as recorded by 24 h recalls, was inversely related to average blood pressure over 6 years, with further positive associations shown for SFA intake and the Key's score(Reference Stamler, Caggiula and Grandits86). Furthermore, plasma cholesteryl ester SFA was positively related and PUFA was inversely related to systolic blood pressure in the Atherosclerosis Risk in Communities (ARIC) 6 years follow-up study(Reference Zheng, Folsom and Ma77).
Chronic intervention studies: blood pressure
Randomised controlled trials that have used methodology to measure arterial compliance and/or endothelial function in order to compare saturated and unsaturated fats (for example, SFA v. MUFA, or MUFA v. n-6 PUFA) are scarce (Table 5). There is some evidence in the literature regarding relative effects of dietary fats on blood pressure(Reference Uusitupa, Sarkkinen and Torpstrom87), but the evidence base is limited by issues such as non-randomisation(Reference Iacono, Puska and Dougherty88, Reference Lahoz, Alonso and Ordovas89), small sample size(Reference Piers, Walker and Stoney90), and reliance on clinic measures of blood pressure rather than ambulatory blood pressure monitoring, which is a more reliable measure of true blood pressure over a 24 h period. There was a trend towards reduced blood pressure following a 4-week MUFA intervention compared with SFA in eight overweight or obese men(Reference Piers, Walker and Stoney90), supported by a larger study in healthy subjects where blood pressure was reduced following a 3-month high-MUFA diet compared with a high-SFA diet, but only in those who consumed < 37 % fat (n 40)(Reference Rasmussen, Vessby and Uusitupa91). Blood pressure was increased following a high-SFA diet compared with a high-MUFA diet but the study design involved non-randomised consecutive 5-week phases of SFA, MUFA, n-6 PUFA, n-6 PUFA plus n-3 PUFA, with no washout periods, and therefore may have been subject to bias(Reference Lahoz, Alonso and Ordovas89). Others have found no differential effects on blood pressure of diets differing in their SFA and PUFA content(Reference Zock, Blijlevens and de Vries92, Reference Sacks, Rouse and Stampfer93), or SFA and MUFA content (The RISCK Study Group, unpublished results).
Table 5 Dietary intervention studies on chronic and acute effects of saturated and unsaturated fatty acids (FA) on blood pressure (BP) and vascular function

M, men; W, women; NR, not randomised; PAL, parallel design; CO, cross-over design; Con, control treatment; LF, low-fat; L-SFA, low-SFA; H-PUFA, high-PUFA; HF, high-fat; H-SFA, high-SFA; HT, hypertensive; H-CHO, high-carbohydrate; LA, linoleic acid; OA, oleic acid; H-MUFA, high-MUFA; AHA, American Heart Association diet; SBP, systolic blood pressure; T2D, type 2 diabetics; ABP, ambulatory blood pressure; SA, stearic acid; PA, palmitic acid; FMD, flow-mediated dilatation; HC, hypercholesterolaemic; NCEP-1, National Cholesterol Education Program-1 diet, low in fat and saturated fat; DBP, diastolic blood pressure; DVP-SI, stiffness index measured by the digital volume pulse; MAP, mean arterial pressure; FBF, forearm blood flow; PWV, pulse wave velocity; DVP, digital volume pulse; AIx, augmentation index.
* Energy intake was also lower on this diet.
† Source: olive oil.
From a public health point of view it would be useful to be able to recommend the relative proportion of MUFA and n-6 PUFA that should be consumed, if saturated fat is less than or equal to the recommended 11 % of food energy intake. There are few examples in the literature where n-6 PUFA has been compared with MUFA with respect to clinic or ambulatory blood pressure or arterial tone. A number of studies found that high-MUFA and high-PUFA diets had no differential effects on clinic blood pressure(Reference Mutanen, Kleemola and Valsta94–Reference Sacks, Stampfer and Munoz97). In contrast, ambulatory blood pressure was reduced, mainly during the day-time, following 3-week high-MUFA diets compared with high-PUFA diets in type 2 diabetics(Reference Thomsen, Rasmussen and Hansen98). These conflicting results may be due to differing methodology for measuring blood pressure or it may be that MUFA is more beneficial than PUFA in the insulin-resistant state only. It has been hypothesised that an increase in ALA intake would decrease blood pressure, since ALA is a precursor of n-3 LCP (known to exert blood pressure-lowering effects). A meta-analysis of ALA and blood pressure studies (total n 348) showed no hypotensive effect, although this was based on three studies only(Reference Wendland, Farmer and Glasziou99). Overall, the results of these studies tend to show that blood pressure is minimally affected by the extent of fatty acid saturation, if at all; however, no firm recommendations can be made with respect to saturated or unsaturated fats and blood pressure until more data from randomised controlled trials with adequate statistical power, using appropriate methodology, are available.
Chronic intervention studies: arterial compliance and endothelial function
There is some evidence in hypercholesterolaemic subjects that changing from a high-SFA diet to a high-MUFA diet can improve FMD, although the dietary MUFA happened to be part of a Mediterranean diet and therefore the role of MUFA per se is unclear(Reference Fuentes, Lopez-Miranda and Sanchez100). The RISCK (Reading University, Imperial College London, Surrey University, MRC Human Nutrition Research Cambridge and King's College London) study also investigated the effects of SFA and MUFA on arterial stiffness (PWV) and endothelium-dependent and endothelium-independent vasodilation (FMD) in a subgroup and, again, no diet-dependent differences were demonstrated. However, arterial stiffness (measured by the DVP method) was marginally reduced following the low-fat diet compared with the high-SFA and high-MUFA diets. Although stiffness index measured by the DVP correlates with PWV (which is mainly a measure of arterial elasticity and age), it is also sensitive to changes in vascular reactivity, suggesting that in this study total fat intake influenced large arterial tone, whereas the dietary fatty acid composition had little influence on vascular function(Reference Sanders, Lewis and Frost101). It is important to note that the RISCK study also investigated the role of high- v. low-glycaemic index diets, and all the comparisons described above were between high-glycaemic index diets. Therefore, it is unknown whether there would have been a difference between SFA and MUFA if a low-glycaemic index diet had been followed.
Keogh et al. reported different findings from their randomised, controlled cross-over trial (n 40) in healthy adults. Comparisons of 3-week diets high in carbohydrate, SFA, MUFA and PUFA showed a marked decrease in FMD following the high-SFA diet compared with the carbohydrate, MUFA and PUFA diets, with no differences observed between the diets high in carbohydrate, MUFA and PUFA(Reference Keogh, Grieger and Noakes102). The difference in findings between these two studies(Reference Sanders, Lewis and Frost101, Reference Keogh, Grieger and Noakes102) may lie in the duration (3 weeks v. 6 months) and type of dietary manipulation that was administered: Keogh et al. (Reference Keogh, Grieger and Noakes102) supplemented the SFA diet with butter only (which is highest in oleic acid, followed by myristic acid (14 : 0) > palmitic acid > stearic acid), whereas Sanders et al. (Reference Sanders, Lewis and Frost101) supplied a range of cooking oils, baking fats, spreads and salad creams which were high in stearic acid, palmitic acid and lauric acid (12 : 0). Although there is currently little evidence that different SFA may differ in their effects on blood pressure(Reference Storm, Thomsen and Pedersen103), this is a relatively unexplored area and until more randomised controlled trials have been carried out in this area, studies that have used different sources of saturated fat may not be strictly comparable.
Acute intervention studies: saturated v. unsaturated fatty acids
With regard to the relative postprandial effects of high-fat meals rich in SFA, MUFA or n-6 PUFA, differences in the source of fat and the type of meal administered in relevant studies published to date preclude any firm conclusions. A high-SFA meal (using shea butter, rich in stearic acid) did not significantly impair FMD after 3 h, whereas a high-MUFA meal (using high-oleic acid sunflower-seed oil) did reduce FMD(Reference Berry, Tucker and Banerji57). The observed reduction in FMD following a MUFA-rich meal was in agreement with previous studies(Reference Ong, Dean and Hayward44, Reference Cortes, Nunez and Cofan58, Reference Vogel, Corretti and Plotnick63). It should be noted, however, that shea butter induces a diminished postprandial lipaemia and oxidative stress compared with high-oleic acid sunflower-seed oil(Reference Berry, Tucker and Banerji57, Reference Berry, Miller and Sanders104). Raitakari et al. also compared a high-SFA meal with a high-MUFA meal, showing no differences in forearm blood flow or FMD between meals; this study is difficult to interpret, however, since the meals were complex (sausages, hash browns and muffins), were not administered in a randomised order, and the MUFA source was not specified(Reference Raitakari, Lai and Griffiths61). Another study compared safflower-seed oil (PUFA-rich) and coconut oil (SFA-rich), showing possible impairment in endothelial function following SFA compared with PUFA, but endothelium-dependent vasodilation differences between fat types were small(Reference Nicholls, Lundman and Harmer60). In summary, acute studies of postprandial vascular response to dietary fat show that a high-fat meal can impair endothelial function, but this is dependent on the other components of the meal, such as protein(Reference Westphal, Taneva and Kastner51), soluble fibre(Reference Katz, Nawaz and Boukhalil105) or antioxidants(Reference Ling, Zhao and Gao64, Reference Katz, Nawaz and Boukhalil105–Reference Esposito, Nappo and Giugliano108); MUFA-rich meals appear to impair endothelium-dependent vasodilation in the brachial artery but the relative effects of different types of fat are still unclear.
Dietary n-3 long-chain polyunsaturated fatty acids: blood pressure, arterial compliance and endothelial function
Consumption of n-3 LCP derived from oily fish has been extensively investigated in relation to CVD risk. A reduction in cardiovascular events and mortality occurs with increased consumption of oily fish(Reference Dokholyan, Albert and Appel109) or following n-3 LCP supplementation(Reference Bucher, Hengstler and Schindler110, Reference Hu and Willett111). The cardioprotective effects of n-3 LCP have been attributed to a number of mechanisms, including effects on blood TAG levels, coagulation, vascular inflammation, heart rate variability, endothelium-dependent vasodilation, arterial tone, eicosanoid balance, blood pressure, cardiac arrhythmia and the stability of the atherosclerotic plaque.
Blood pressure
The blood pressure-lowering effects of n-3 LCP are well established(Reference Mori112), and the epidemiological evidence for an inverse relationship between n-3 LCP intake and blood pressure(Reference Ueshima, Stamler and Elliott113) is supported by data from dietary trials. Blood pressure can be reduced by an average of 2·3 mmHg (systolic blood pressure) and 1·5 mmHg (diastolic blood pressure) following increased n-3 LCP intake, as shown in a meta-analysis of thirty-six randomised trials that had administered fish oil to hypertensives and non-hypertensives, with doses ranging from 0·2 to 15 g/d (median 3·7 g/d)(Reference Geleijnse, Giltay and Grobbee114). The reduction in blood pressure was more pronounced in older subjects. It is not known whether this effect is attributable to EPA and DHA together, or just one of them, but a recent study showed that a moderate dose of 0·7 g DHA/d lowered diastolic blood pressure by 3·3 mmHg(Reference Theobald, Goodall and Sattar115), whereas EPA does not seem to be effective in reducing blood pressure(Reference Cazzola, Russo-Volpe and Miles116, Reference Mori, Bao and Burke117).
Arterial compliance and endothelial function
Arterial stiffness is reduced in populations that have increased intakes of n-3 LCP(Reference Hamazaki, Urakaze and Sawazaki118). Supplementation with n-3 LCP at doses of 1·8–3 g/d for 6 weeks to 12 months improved arterial compliance and PWV in type 2 diabetics and dyslipidaemics(Reference McVeigh, Brennan and Cohn119–Reference Tomiyama, Takazawa and Osa121). However, supplementation with 0·7 g DHA/d had no effects on the arterial stiffness index or the reflected wave using DVP(Reference Theobald, Goodall and Sattar115), suggesting either that higher doses are required to have a physiological effect or that EPA is the bioactive component of fish oil which improves the elasticity of the artery. The evidence for differential effects of EPA and DHA on blood pressure is conflicting. EPA supplementation (1·8 g/d, approximately 2 years) reduced arterial stiffness in type 2 diabetics, probably by improving arterial integrity since carotid intima-media thickness was also reduced, although no changes in blood pressure were observed(Reference Mita, Watada and Ogihara122). Tomiyama et al. also detected a beneficial effect on arterial stiffness over 12 months with EPA only, and it was suggested that this fatty acid may reduce PWV by modulating eicosanoid metabolism(Reference Tomiyama, Takazawa and Osa121). However, despite a slightly greater increase in systemic arterial compliance following 3 g EPA/d compared with DHA (36 v. 27 % respectively), there was no significant difference between the two treatments(Reference Nestel, Shige and Pomeroy120). A dose–effect threshold and/or duration of treatment are possibly stronger determinants of the effects of chronic n-3 LCP intake on arterial stiffness, rather than the type of fatty acid.
Early studies using animal models demonstrated that endothelial function could be modulated by feeding EPA and DHA(Reference Mark and Sanders123–Reference Shimokawa and Vanhoutte125). Cross-sectional evidence indicated that dietary EPA and DHA intakes are positively associated with endothelial function in young smokers (but not non-smokers) and young adults at greater metabolic risk(Reference Leeson, Mann and Kattenhorn126). Estimates of dietary n-3 LCP intake are also inversely associated with markers of endothelial activation, for example, cell adhesion molecules(Reference Lopez-Garcia, Schulze and Manson127). Supplementation with n-3 LCP (EPA plus DHA) for periods ranging from 2 weeks up to 8 months improved endothelium-dependent vasodilation, prevented vasoconstriction or augmented exercise-induced blood flow at doses ≥ 0·5 g/d(Reference Chin, Gust and Nestel128–Reference Walser, Giordano and Stebbins136). A moderately low dose of DHA alone did not have much effect on salbutamol-induced changes in reflection index measured by the DVP, but this may be due to methodological problems in detecting endothelium-dependent vasodilatory responses using the digital pulse contour analysis technique(Reference Theobald, Goodall and Sattar115).
Recently, a handful of studies have addressed the acute mechanisms whereby n-3 LCP may influence arterial tone. Large arterial tone was reduced following two sequential high-fat meals when the initial meal contained 5 g EPA compared with high-fat control meals, possibly due to NO-independent mechanisms, since it was also observed that plasma NO metabolite (NOx) concentrations were not influenced by EPA consumption(Reference Hall, Sanders and Sanders137). Consumption of tinned red salmon or rapeseed oil (containing 6 and 5 g n-3 PUFA respectively) resulted in no postprandial change in FMD, whereas an olive oil meal containing an equal amount of fat significantly impaired FMD(Reference Vogel, Corretti and Plotnick63); this could be interpreted as a prevention of impaired NO production by n-3 LCP following the salmon meal, although it is equally as likely to be the presence of protein(Reference Westphal, Taneva and Kastner51). FMD was improved postprandially when the meal contained n-3 PUFA (either EPA/DHA or ALA) in type 2 diabetics who had high fasting plasma TAG but not in type 2 diabetics with normal fasting TAG(Reference West, Hecker and Mustad138, Reference Hilpert, West and Kris-Etherton139). Other researchers demonstrated an acute effect of fish oil on endothelium-independent vasodilation in the microvasculature using laser Doppler iontophoresis but no significant change in endothelium-dependent vasodilation(Reference Armah, Jackson and Doman140), possibly indicating differential mechanisms for n-3 LCP-induced postprandial vasodilation according to the size and location of the blood vessel. The lack of studies that have investigated acute effects of n-3 PUFA on arterial tone precludes any clear idea of the relative dose-related effects of EPA and/or DHA on vascular function. However, it may be tentatively suggested that these fatty acids may induce vasorelaxation postprandially, potentially via both endothelium-dependent and endothelium-independent routes.
Mechanisms for modulation of vascular function and blood pressure by dietary fatty acids
The key findings from the review of the epidemiological and intervention studies can be summarised simply: (1) long-term total dietary fat intake probably does not influence vascular function or blood pressure independently of the saturated fat content; (2) biomarkers of dietary saturated fat intake are positively associated with blood pressure, although a causal effect has not been confirmed by robustly designed randomised controlled trials; (3) high-fat meals may impair postprandial endothelial function, particularly high-MUFA meals; however, there is no evidence that long-term consumption of high-MUFA diets have any adverse effect on blood pressure or vascular function; (4) higher levels of dietary LA and ALA intake are associated with improved blood pressure but there is not enough randomised controlled trial evidence to draw definite conclusions; and (5) chronic studies suggest that n-3 LCP can reduce blood pressure, improve arterial compliance in type 2 diabetics and dyslipidaemics, and augment endothelium-dependent vasodilation, but few studies have investigated acute effects.
Although there is not yet enough evidence from human trials regarding the effects of fatty acid saturation and vascular function, there is evidence in the rat that a high-SFA diet increases systolic blood pressure, with the opposite effect induced by a high-LA diet(Reference Langley-Evans, Clamp and Grimble141). The possible mechanisms for the opposing effects of dietary SFA and n-6 PUFA intake are not yet clear. Studies in vitro have shown that incubation of cultured human coronary artery endothelial cells and smooth muscle cells with palmitate caused an increased expression of the inflammatory cytokine, IL-6; this did not occur when linoleate was added(Reference Staiger, Staiger and Stefan142). The increase in IL-6 expression was especially marked in the endothelial cells, suggesting that dietary palmitic acid may increase blood pressure by a pro-inflammatory mechanism within the endothelium. It has been clearly demonstrated that elevated circulating levels of NEFA are associated with higher blood pressure(Reference Sarafidis and Bakris143) and can induce endothelial dysfunction of conduit arteries(Reference Steinberg, Paradisi and Hook144, Reference Steinberg, Tarshoby and Monestel145). Circulating NEFA reflect dietary fatty acid composition to some extent, so it seems likely that a high dietary palmitic acid intake, especially in obese or insulin-resistant individuals (who are likely to have elevated circulating NEFA), could impair vascular function and raise blood pressure. Elevated SFA-rich NEFA may cause concomitant endothelial dysfunction and insulin resistance by inhibition of the common insulin-mediated cell-signalling pathway that activates eNOS in endothelial cells and triggers GLUT-4 translocation in skeletal muscle cells(Reference Kim, Tysseling and Rice146, Reference Muniyappa and Quon147). In addition to their potential pro-inflammatory effects, SFA may also impair vascular function by increasing oxidative stress within the endothelium, as evidenced by the reduction in SFA-induced impairment in endothelium-dependent vasodilation of rat resistance arteries and rabbit aorta by the addition of ascorbic acid or superoxide dismutase(Reference Sainsbury, Sattar and Connell148, Reference Edirisinghe, McCormick and Kappagoda149). Furthermore, the latter study showed that the SFA-induced impairment of endothelium-dependent vasodilation occurred acutely (within 15 min) and was associated with NO production(Reference Edirisinghe, McCormick and Kappagoda149).
The impact of high-fat meals on postprandial vascular function has often been suggested to be linked with postprandial lipaemia. The size of the increase in postprandial TAG is inversely associated with the decrease in endothelial function(Reference Marchesi, Lupattelli and Schillaci55, Reference West, Hecker and Mustad138, Reference Anderson, Evans and Ellis150), and a greater impairment in FMD following a high-fat meal is observed in hypertriacylglycerolaemics compared with normolipidaemics(Reference Norata, Grigore and Raselli151). Postprandial TAG-rich lipoproteins up-regulated the expression of cell adhesion molecules, monocyte chemoattractant protein-1 and IL-6 when incubated ex vivo with endothelial cells, indicating a probable endothelial inflammatory response to high-fat meals in vivo (Reference Norata, Grigore and Raselli151), especially those isolated following a high-SFA meal(Reference Williams, Maitin and Jackson152). The acute effect of postprandial lipaemia on endothelial function is clearly somehow linked to increased oxidative stress(Reference Bae, Schwemmer and Lee42, Reference Tsai, Li and Lin49, Reference Tushuizen, Nieuwland and Scheffer50, Reference Katz, Nawaz and Boukhalil105, Reference Plotnick, Corretti and Vogel106, Reference Esposito, Nappo and Giugliano108, Reference Anderson, Evans and Ellis150, Reference Nappo, Esposito and Cioffi153), but the precise mechanisms remain to be discovered. One theory proposes that remnant TAG-rich lipoproteins induce production of reactive oxygen species within the endothelium, reducing the bioavailability of NO. Remnant TAG-rich lipoproteins isolated from hyperlipidaemic subjects impaired endothelium-dependent relaxation in rabbit aortic strips(Reference Doi, Kugiyama and Ohgushi154) and levels of remnant TAG-rich lipoproteins were associated with impaired dilation of coronary arteries in human subjects, linked to NO bioavailability(Reference Kugiyama, Doi and Motoyama155). Some recent research using artificial chylomicron remnant-like particles has helped to resolve the molecular mechanisms that mediate postprandial effects on the endothelium, showing that these particles inhibit NO production from cultured endothelial cells, induce cyclo-oxygenase-2 activity, and increase expression of inflammatory molecules and antioxidant enzymes(Reference Wheeler-Jones156).
The likely mechanisms for the beneficial effects of n-3 LCP on the arterial wall are numerous and may differ for DHA and EPA. In vitro studies have been employed to investigate the effects of different fatty acids on endothelial cells but differences in experimental conditions make the evidence extremely difficult to interpret, as outlined by Shaw et al. in a recent attempt to compare the effects of a wide range of fatty acids on endothelial function in human umbilical vein endothelial cells(Reference Shaw, Hall and Jeffs157). However, broad patterns that emerge from this literature indicate that DHA has a greater effect than EPA in reducing endothelial inflammation. DHA tends to inhibit markers of endothelial function, such as inflammatory cell adhesion molecules and monocyte chemoattractant protein-1 gene and protein expression, and the adhesion of leucocytes to the endothelium, whereas EPA either up-regulated gene expression of monocyte chemoattractant protein-1 or was a weaker inhibitor of cell adhesion molecules than DHA(Reference Shaw, Hall and Jeffs157–Reference Weber, Erl and Pietsch161).
Interestingly, the extent of inhibition of vascular cell adhesion molecule-1 by DHA was directly related to the increase in incorporation of DHA into the total endothelial cell lipids, possibly into the phospholipids of the inner plasma membrane(Reference De Caterina and Massaro158). One of the mechanisms by which EPA and DHA are believed to improve vascular tone are by increasing endothelial cell membrane fluidity, and in fact DHA appears to be the more effective of the two in increasing plasma membrane fluidity of vascular endothelial cells(Reference Hashimoto, Hossain and Yamasaki162). In addition, DHA treatment increases the total n-3 LCP content of caveolae (invaginations of the plasma membrane where eNOS is located when it is inactivated) in cultured endothelial cells, and displaces the protein caveolin-1, which is responsible for securing eNOS in the caveolae region, from the caveolae(Reference Li, Zhang and Wang163). This suggests that DHA may influence endothelial function by incorporation into the cell membrane, including caveolae, leading to an increase in eNOS activity and an inhibition of intracellular signalling pathways that may activate the inflammatory response(Reference Chen, Jump and Esselman164). In fact the relationship between DHA and caveolin-1 and eNOS distribution in the endothelial cell membrane was also demonstrated with EPA(Reference Li, Zhang and Wang165), and incubation with both EPA and DHA can induce eNOS translocation and NO production in cultured endothelial cells(Reference Li, Zhang and Wang163, Reference Li, Zhang and Wang165–Reference Omura, Kobayashi and Mizukami167).
Another mechanism whereby EPA and DHA may influence vascular tone is via modulation of the eicosanoid pathway. EPA and DHA inhibit cyclo-oxygenase-1 protein and gene expression in cultured endothelial cells, thereby inhibiting the conversion of arachidonic acid to PG, some of which is eventually converted to thromboxane, a vasoconstrictor, and some to PGI2, a vasodilator(Reference Achard, Gilbert and Benistant168, Reference Gilbert, Dalloz and Maclouf169). However, incubation with EPA and DHA increases PGI2 and PGI3 production in vitro and in vivo (Reference Abeywardena, Fischer and Schweer170, Reference Hishinuma, Yamazaki and Mizugaki171), PGI3 being an EPA-derived PGI which acts as a vasodilator similarly to PGI2. EPA-derived epoxyeicosatrienoic acids, which can be synthesised in the endothelium by P450-dependent epoxygenation of EPA instead of arachidonic acid, and are thought to be an endothelium-derived hyperpolarising factor, may influence vascular tone via modulation of Ca-activated K channels in vascular smooth muscle cells(Reference Lauterbach, Barbosa-Sicard and Wang172). Finally, the oxidation of EPA to F3-isoprostanes may also result in reduced oxidative stress and increased vasorelaxation(Reference Gao, Yin and Milne173).
Summary and conclusions
In summary, consideration of the epidemiological evidence suggests an adverse effect of SFA and a beneficial effect of LA and ALA on vascular function, but the quantity and quality of the experimental evidence is not sufficient to support this convincingly. Reports of differential effects of saturated and unsaturated fatty acids on blood pressure and vascular function are patchy and inconclusive, and it would be premature to make any recommendations based on the literature to date. Large, well-powered randomised controlled trials are required to determine the differential effects of dietary fatty acid composition on blood pressure, arterial compliance and endothelial function in men and women, both in the healthy state and with metabolic or vascular complications. The strength of evidence that high-fat meals transiently impair shear stress-induced endothelium-dependent vasodilation is overwhelming, but the exact intracellular events that mediate these effects are not fully understood. Currently the impairment in endothelium-dependent vasodilation during postprandial lipaemia appears to be associated with an induction of pro-inflammatory pathways and oxidative stress, with elevated NEFA and postprandial TAG-rich lipoproteins being potential mediators in this process. These acute effects on arterial function should not be underestimated, as a lifetime of high-fat meals and transient endothelial injury could ultimately accumulate into major arterial damage, and much remains to be understood regarding the differential acute effects of saturated and unsaturated fatty acids. The role of n-3 LCP in vascular health is well known and the quality of the evidence for an effect of EPA and DHA on blood pressure, arterial compliance and endothelial function is much stronger than that for SFA, MUFA, ALA and n-6 PUFA. However, more studies investigating the differential effects of EPA and DHA, following both long-term consumption and acute doses, will shed more light on the mechanisms for their beneficial effects, and will furnish health professionals and nutritionists with a greater knowledge base from which to make recommendations to the general public.
Acknowledgements
I have no conflicts of interest to declare.








