Introduction
Inorganic nitrate has traditionally been considered as an inert contaminant in food and water. This anion is formed as part of the N cycle by the action of N-fixing bacteria in plants and therefore vegetables are the main source of nitrate in our diet. However, the opinion of this natural compound has substantially changed over the last decade, as a result of new evidence linking dietary nitrate consumption with reduction of blood pressure and potential benefits to cardiovascular health. This is significant since hypertension affects 25 % of the world population and is considered a major risk factor for CVD, renal disease and dementia. In the UK, approximately 30 % of the adult population have high blood pressure( 1 ). A small reduction of blood pressure across the population in England alone could save 45000 quality-adjusted life years over 10 years, in addition to £850 million not spent on health and social care every year( 2 ). Thus, new approaches to reduce the prevalence of high blood pressure across the population are urgently required. This review will summarise historical aspects and current understanding of the role of dietary nitrate as a potential nutrient in the diet to reduce blood pressure and promote cardiovascular health.
Inorganic nitrate in food and water
The circulatory levels of inorganic nitrate are determined by endogenous (NO synthase (NOS) enzymes) and exogenous (diet) sources. It has been estimated that up to 50 % of human plasma nitrate is derived from dietary sources( Reference Hord, Tang and Bryan 3 ). Vegetables (defined as a plant or part of a plant used as food) are the main source of this anion although important variations can be found amongst different plants. For instance, green leafy vegetables such as rocket, spinach, kale, pak choy and certain types of lettuce have the highest levels of nitrate, with up to 480 mg/100 g( 4 ). In contrast, the nitrate content of legumes such as peas can be as low as 3 mg/100 g( 4 ) (Table 1)( 4 – 8 ). Not only does nitrate content vary between species, but also within species, so that mature outer leaves have higher concentrations than younger inner leaves.
Table 1 Nitrate content of vegetables, portion size and nitrate content per portion
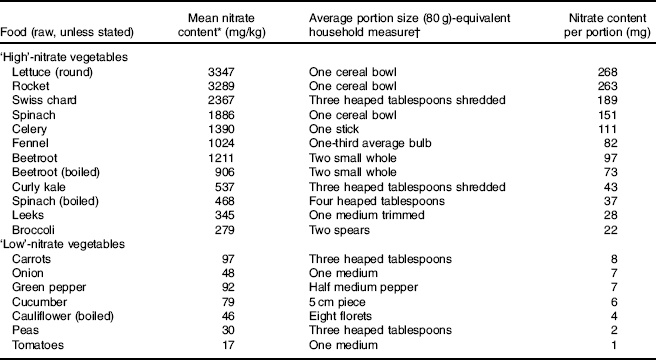
* Data obtained from the European Food Safety Authority( 4 ), Ministry of Agriculture, Fisheries and Food( 5 ), Santamaria et al. ( Reference Santamaria, Elia and Serio 6 ) and the Food Standards Agency( 7 ).
† Data from NHS Choices( 8 ).
In addition to inter- and intra-species variations, environmental factors significantly affect nitrate content of vegetable crops. For example, nitrate levels increase if crops are grown in low light conditions, such as in dull or cloudy weather or when grown under cover. Therefore there are also seasonal differences, so that the nitrate content of vegetables harvested in autumn and winter can be significantly higher than those harvested in spring and summer( Reference Santamaria, Elia and Serio 6 ). Natural light activates the enzyme nitrate reductase, which converts nitrate to nitrite, so nitrate accumulates more in low light conditions( Reference Weightman, Hucklea and Roquesa 9 ). Other environmental factors include the amount of water received by the vegetable crops: too little water restricts nitrate uptake whereas too much dilutes nitrate in the soil. The amount of organic matter such as animal manure in the soil, as well as the amount of N fertiliser applied also affects nitrate content( 4 ). Vegetables produced from soil high in organic matter, even where no chemical fertiliser is applied, can have higher nitrate content than the same species grown in chemically fertilised soil( Reference Bryan and Loscalzo 10 ).
Once harvested, nitrate content can be further affected by storage and cooking. For example, refrigeration or freezing of vegetables prevents nitrate loss, but storage at ambient temperature increases nitrate losses( 4 ). As nitrate is soluble, it can be lost by washing, peeling and boiling. For example, boiling vegetables can reduce nitrate content by up to 75 %( 5 ), whereas steaming retains nitrate content( Reference Mozolewski and Smoczynski 11 ).
To a lesser extent, water and food additives are also sources of nitrate in the diet. Levels of nitrate in drinking water supplies in the UK and other developed countries are strictly controlled by legislation at a maximum of 50 mg/l( 12 ). Due to the high solubility of nitrate, water supplies can be contaminated by overuse of nitrate-containing fertilisers, which are subsequently washed off agricultural land into streams, rivers and lakes. Excessive amounts of nitrate have been detected in water from 22 % of privately owned wells in the USA( Reference Ward, Kok and Levallois 13 ). Guidelines for nitrate in drinking water were mainly promulgated to protect bottle-fed infants from developing methaemoglobinaemia, or cyanosis. Higher concentrations of nitrate in blood are associated with oxidation of Hb in erythrocytes and, if the level approaches 20 % of total Hb, methaemoglobinaemia can develop which interferes with blood transport of oxygen( Reference Bryan and Loscalzo 10 ). The risk of this condition in infants under 12 months old is higher than in adults, because of immaturity of their enzymic systems and the low acid production capacity of their stomachs. Higher gastric pH enables growth of intestinal flora which convert nitrate to nitrite, which is directly absorbed into the blood( Reference Fewtrell 14 ). However, further analysis offers a more complex picture of the causes of infantile methaemoglobinaemia, suggesting that gastrointestinal infection and inflammation as well as overproduction of endogenous NO may be the cause of this condition( Reference Avery 15 ). Furthermore, other compounds such as vitamin C appear to be a protective factor against methaemoglobinaemia in infants( Reference Ward, Kok and Levallois 13 ). Current opinion suggests that given the apparently low incidence of possible water-related methaemoglobinaemia, it is inappropriate to attempt to link the concentration of nitrate in drinking-water to methaemoglobinaemia( Reference Fewtrell 14 ).
Nitrate (and nitrite) salts are used as food additives due to their ability to inhibit the growth of Clostridium botulinum bacterial spores. For example, sodium and potassium nitrate, together with sodium chloride, are used in ‘curing’ solutions during the production of bacon and ham( 16 ). Nitrite in combination with salt and other curing factors may also control the growth of other pathogens such as Bacillus cereus, Staphylococcus aureus and Clostridium perfringens ( Reference Hord, Tang and Bryan 3 ). Other interesting properties of nitrite salts when added to meat products include the reduction and reaction with myoglobin to produce the characteristic reddish-pink colour, in addition to contribution to the cured flavour and inhibition of lipid oxidation. However, cured meats are minor contributors to total dietary nitrate intake compared with vegetables, as detailed below.
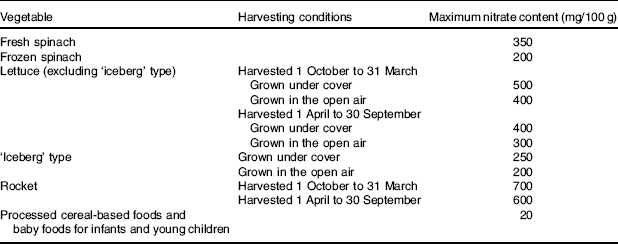
The prevailing opinion is that dietary nitrate from all sources consumed in excess of the acceptable daily intake (ADI) of 3·7 mg/kg per d is harmful to human health and this is reflected in UK( 7 ) and European Community legislation( 4 ). An ADI is the ‘amount of a food additive, expressed as mg/kg body weight that can be ingested daily over a lifetime without incurring any appreciable health risk’( 17 ). Although the toxicity of nitrate is low, the oral lethal dose of nitrate for humans has been reported at about 330 mg/kg per d (equivalent to about 23100 mg for a 70 kg adult/d)( Reference Walker 18 ). Due to a lack of new data, the European Food Safety Authority accepted the previous ADI set by the Joint FAO/WHO Expert Committee on Food Additives in 2002( 4 ). To ensure that intakes remain below the ADI, maximum levels of nitrate in crops such as lettuce, spinach and rocket are defined by legislation( 19 ) (Table 2). Similar restrictions apply to the addition of potassium and sodium nitrate to meat products, such as bacon and ham( 20 ). In addition, the agriculture industry must adhere to separate legislation to minimise the use of nitrate fertilisers in order to avoid contaminating the water supply, for example, by providing storage for animal waste( 21 ). Therefore, the view of nitrate as a harmful contaminant has significant implications for the food, agriculture and water industries today.
Table 2 European Commission regulations on maximum levels of nitrate in foods (adapted from Commission Regulation (EU) no. 1258/2011)( 19 )
Dietary nitrate consumption
Given that humans consume the majority of nitrate from vegetables, assessment of vegetable intake has been used as an indicator of nitrate intake. For example, a recent Dutch prospective cohort study of men and women, aged 55–69 years, estimated nitrate intake by using a FFQ together with local nitrate data (Dutch State Institute for Quality Control of Agricultural Products)( Reference Keszei, Schouten and Driessen 22 ). Taking into account seasonal differences and factoring in losses from cooking and preparation, these authors estimated the median nitrate intake from vegetables to be approximately 100 mg/d (range 74–124 mg/d). In contrast, the median intake from cured meats, such as bacon and ham, is approximately 5 mg/d( Reference Hsu, Arcot and Lee 23 ) and from water is about 1 mg/d( Reference Keszei, Schouten and Driessen 22 ). In the UK, the average consumption of vegetables (including potatoes, beans and pulses) by adults aged 19–64 years taking part in the National Diet and Nutrition Survey is about 220 g/d( 24 ). Taking a mean nitrate concentration of all vegetables, including potatoes, as 33·6 mg/100 g( 4 ), the UK current nitrate intake from all vegetables can be estimated as approximately 70 mg/d. This is in line with previous figures reported by the UK Ministry of Agriculture, Fisheries and Food, who estimated the mean dietary nitrate intake (excluding water and beer) as 57 mg/d (upper limit 105 mg/d)( 5 ) and 54 mg/d reported by Gangolli et al.( Reference Gangolli, van den Brandt and Feron 25 ). In addition, it is consistent with estimated nitrate intake from vegetables reported from other countries, which range from 71 mg (Finland) to 134 mg/d (Italy)( Reference Santamaria, Elia and Serio 6 ). These estimates of nitrate intake are within the ADI for nitrate of 3·7 mg/kg per d or about 260 mg/d for a 70 kg adult.
Whilst worldwide production of fruits and vegetables has been increasing over recent years, inadequate consumption remains a problem in the UK, where only 30 % of adults achieve the ‘5-A-Day’ target( 24 ). Bryan( Reference Bryan 26 ) noted that the typical Western diet will be low in nitrate as it is high in refined cereals, refined sugars, dairy products, refined vegetable oils, salt and fatty meats. Conversely, those with the highest vegetable intake such as vegetarians can potentially have the highest nitrate intakes, depending on food preferences. For instance, the nitrate intake of vegetarians in the UK has been estimated as three times greater than the rest of the population( Reference Gangolli, van den Brandt and Feron 25 ) and could be up to 268 mg/d( Reference Lidder and Webb 27 ). Similar data have been reported from vegetarians in Poland showing an average consumption of nitrate nearly three times higher (mean 340 mg/d, range 37–2054 mg/d) than non-vegetarians (mean 125 mg/d, range 116–134 mg/d)( Reference Mitek, Anyzewska and Wawrzyniak 28 ). However, the range of nitrate ingestion might differ substantially among vegetarians due to food choices and the wide variation of nitrate content in different plants and fruits. For instance, Hord et al.( Reference Hord, Tang and Bryan 3 ) quantified the nitrate concentration of two hypothetical diets that emphasised low-nitrate and high-nitrate vegetables based on the Dietary Approach to Stop Hypertesion (DASH) diet. They found that nitrate concentrations in these diets varied from 174 to 1222 mg, respectively( Reference Hord, Tang and Bryan 3 ). Thus, these data suggest that different patterns in the consumption of vegetables can yield differences in nitrate intake of over 700 % and can be significantly greater than the ADI. It has also been suggested that the Mediterranean diet, well known for its cardioprotective properties, might provide substantial amounts of nitrate because of its high content of nitrate-rich vegetables( Reference Bryan and Loscalzo 10 , Reference Lidder and Webb 27 ). However, no previous study has quantified the nitrate content of the Mediterranean diet to date. In addition, prospective cohort studies suggest that green leafy vegetables have protective effects against CVD( Reference Joshipura, Hung and Li 29 ) but have not assessed nitrate intake.
Metabolism of dietary nitrate
Traditionally, inorganic nitrate was associated with an increased risk of gastrointestinal cancer and methaemoglobinaemia in infants and so considered as a contaminant in vegetables and water. This link was supported by animal research performed between the 1960s and 1970s that suggested a link between dietary nitrate and carcinogenesis( Reference Correa, Haenszel and Cuello 30 , Reference Bryan, Alexander and Coughlin 31 ). More specifically, it was reported that dietary nitrate consumption could lead to the formation of endogenous N-nitrosamines, most of which were shown to be carcinogens in rodents. Following these previous studies and the above data, it might be expected that the incidence of gastric cancer in vegetarians would be higher due to larger consumption of dietary nitrate. However, epidemiological studies in this population have shown the opposite effect, as vegetarian diets are associated with low mortality rates( Reference Key, Appleby and Spencer 32 ) and are protective against CVD and different types of cancer( Reference Huang, Yang and Zheng 33 ). In accordance with this, Bryan et al. ( Reference Bryan, Alexander and Coughlin 31 ) indicated that the original nitrate/gastric cancer hypothesis was based on low-quality studies and has not been confirmed by more recent animal and epidemiological studies. Furthermore, a recently published meta-analysis on this issue found that a high nitrate intake was associated with a weak but statistically significant reduced risk of cancer in humans( Reference Song, Wu and Guan 34 ). Therefore, current evidence for adverse effects of moderate dietary nitrate consumption above the ADI is weak.
On the other hand, research performed over the last decade has indicated that dietary nitrate could be viewed as a bioactive food component. Briefly, current evidence suggests that dietary nitrate is rapidly absorbed from the gastrointestinal tract into the circulation with plasma levels remaining high for 5–6 h after ingestion( Reference Lundberg, Weitzberg and Gladwin 35 ). Subsequently, part of this nitrate is actively taken up by the salivary glands, concentrated up to twenty times higher than plasma levels and secreted into the oral cavity in the saliva( Reference Lundberg, Weitzberg and Gladwin 35 ). Oral bacteria reduce salivary nitrate to nitrite which is swallowed and absorbed across the upper gastrointestinal tract and into the circulation( Reference Lundberg, Weitzberg and Gladwin 35 ). It is not known how nitrite crosses the gut wall into the circulation, but there have been suggestions that anion exchanger 1 in erythrocytes may be involved( Reference Vitturi, Teng and Toledo 36 ). When swallowed, the acidic environment of the stomach facilitates the reduction of nitrite to NO and other N forms( Reference Lundberg and Govoni 37 ). This reaction is enhanced by the presence of vitamin C and polyphenols in the stomach( Reference Lidder and Webb 27 ), both of which can be obtained from vegetables. Circulatory nitrite can also be reduced to NO through the activity of a number of enzymes with nitrite reductase capability present in several cells such as Hb, myoglobin and mitochondria( Reference Lundberg, Weitzberg and Gladwin 35 ).
The key role of oral bacteria in the conversion of dietary nitrate to nitrite has been shown in recent studies using antiseptic mouthwash( Reference Govoni, Jansson and Weitzberg 38 – Reference McDonagh, Wylie and Winyard 41 ). These studies found that disruption of the nitrate-reducing capacity of oral bacteria by oral mouthwash was associated with no change in plasma circulatory levels of nitrite after nitrate intake. In addition, this response was linked to a concomitant increase in blood pressure( Reference Kapil, Haydar and Pearl 39 ), demonstrating a correlation between oral bacteria, dietary nitrate ingestion, increased circulatory levels of nitrite and reduced blood pressure.
The identification of this bioactive role of dietary nitrate has substantially changed the view of NO metabolism. In the past it was thought that NO was only formed via an enzymic and oxygen -dependent pathway, where l-arginine is reduced to NO by several forms of NOS enzymes and the combination of several cofactors( Reference Moncada and Higgs 42 ). In addition, it was found that patients with hypertension have impaired NO bioavailability, caused by increased levels of reactive oxygen species which scavenge NO( Reference Hermann, Flammer and Lüscher 43 ). This reduced NO bioavailability is a major risk factor for both hypertension and CVD( Reference Hermann, Flammer and Lüscher 43 ). Consequently many studies in the last two decades have investigated the effects of l-arginine supplementation and NO bioavailability in patients with hypertension. A meta-analysis of these studies found that supplementation with l-arginine was effective in reducing blood pressure( Reference Dong, Qin and Zhang 44 ). However, it must be noted that the amount of l-arginine provided by some studies was very high (>20 g/d) compared with the amount of l-arginine in the normal diet (about 5 g/d)( Reference Venho, Voutilainen and Valkonen 45 ). This is important since large amounts of oral l-arginine have been reported to increase risk of mortality in both animals with septic shock and patients with acute myocardial infarction( Reference Kalil, Sevransky and Myers 46 , Reference Schulman, Becker and Kass 47 ).
Thus, current research is now focusing on the effect of dietary nitrate on NO metabolism and vascular function. From this viewpoint, a study by Carlström et al. ( Reference Carlström, Persson and Larsson 48 ) found that nitrate administration was able to increase bioavailability and reverse cardiovascular dysfunction in mice with impaired function of the l-arginine–NOS pathway. However, these results have not been confirmed in longer-term studies( Reference Hezel, Liu and Schiffer 49 ) and there is a possibility of a cross-talk between the NOS-dependent and NOS-independent pathways in animals( Reference Carlström, Liu and Yang 50 ). In addition there is a lack of long-term supplementation studies in human subjects and future studies should explore the effects of high intakes of dietary nitrate (>ADI) on the oral microbiome and blood pressure.
Is dietary nitrate a new nutrient?
Since the first human study showing a blood pressure-lowering effect of a dietary nitrate supplement was published in 2006( Reference Larsen, Ekblom and Sahlin 51 ), many studies have extended these findings. A systematic review and meta-analysis of such studies published from 2006 to 2013 found that nitrate supplements reduced systolic blood pressure by 4·4 mmHg and diastolic blood pressure by 1·1 mmHg( Reference Siervo, Lara and Ogbonmwan 52 ). Participants included a mixture of adults with and without health co-morbidities who were given pharmacological (sodium or potassium nitrate) or dietary (beetroot juice) supplements. Similarly, a review by Hobbs et al. ( Reference Hobbs, George and Lovegrove 53 ) of studies up to 2012 found a significant inverse relationship between nitrate dose and systolic blood pressure. In agreement with these results, seventeen further intervention studies have been published( Reference Ghosh, Kapil and Fuentes-Calvo 54 – Reference Jonvik, Nyakayiru and Pinckaers 70 ) (Table 3). These studies found significant reductions in blood pressure after various forms of nitrate supplementation (mean dose about 500 mg nitrate) in a wide range of participants, including patients with hypertension, chronic obstructive pulmonary disease and heart failure. Together with previous reviews( Reference Siervo, Lara and Ogbonmwan 52 , Reference Hobbs, George and Lovegrove 53 ), these more recent studies support the hypothesis that dietary nitrate reduces blood pressure and therefore could improve cardiovascular health. It could be concluded that there are sufficient data to state that dietary nitrate, at least from vegetables, should be reconsidered as a new nutrient, rather than a harmful contaminant( Reference Hord, Tang and Bryan 3 ).
Table 3 Summary of research studies on nitrate and health with significant (P<0·05) positive effects on systolic and diastolic blood pressure, 2013–2016
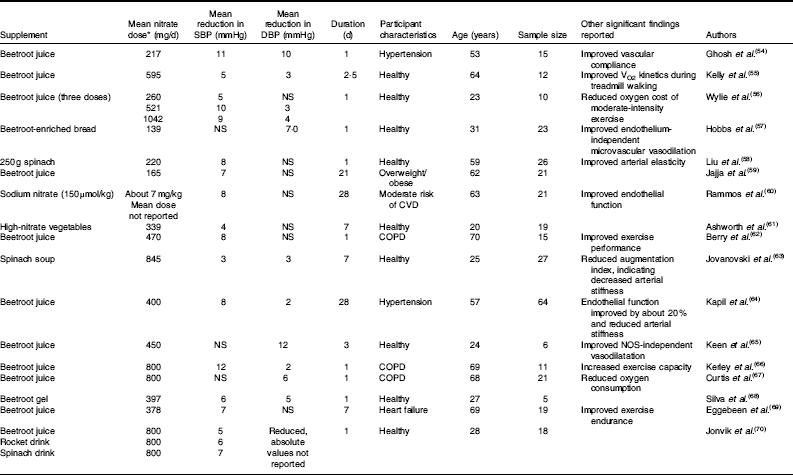
SBP, systolic blood pressure; DBP, diastolic blood pressure; COPD, chronic obstructive pulmonary disease; NO, NO synthase.
* Mean nitrate dose of all studies about 500 mg/d, range 139–1042 mg/d.
Accordingly, it has been suggested that the key element of the cardioprotective effect of diets rich in vegetables is dietary nitrate( Reference Hord, Tang and Bryan 3 ). Interestingly, the prevalence of high blood pressure in vegetarians is significantly lower than in omnivores as shown by epidemiological studies( Reference Huang, Yang and Zheng 33 , Reference Key, Thorogood and Appleby 71 ). Originally it was thought that the lower energy density, higher fibre and lower fat content of vegetarian diets induced low BMI and this could explain low values of blood pressure in vegetarians compared with omnivores( Reference Drøyvold, Midthjell and Nilsen 72 ). However, weight differences do not fully explain the observed blood pressure differences between vegetarians and omnivores. Pettersen et al.( Reference Pettersen, Anousheh and Fan 73 ) found that differences in systolic blood pressure (about 6–7 mmHg) between vegans and lacto-ovo vegetarians compared with omnivores remained after adjusting the data for age, sex and BMI. These authors also suggested that various factors such as the higher fibre and K intakes in vegetarians may be responsible for this effect on blood pressure, along with possible physiological mechanisms, such as improvement of glucose tolerance and lower blood viscosity( Reference Pettersen, Anousheh and Fan 73 ). However, positive effects of K or Mg supplements on blood pressure have not been confirmed and these are not recommended for treatment of hypertensive patients( Reference Dickinson, Nicolson and Campbell 74 , Reference Dickinson, Nicolson and Campbell 75 ). Low ingestion of Na can also be associated with low blood pressure as suggested by several studies in an attempt to explain the effects of the DASH diet( Reference Sacks, Svetkey and Vollmer 76 , Reference Vollmer, Sacks and Ard 77 ). There is still an intense debate on the main factors associated with low blood pressure, i.e. whether it is the reduced salt content associated with vegetable-rich diets, or other constituents of foods such as nitrate or polyphenols. From this viewpoint, we are currently undertaking a clinical trial to investigate the contribution of dietary nitrate in lowering blood pressure in individuals following a vegetarian diet for at least a year. This and further studies are needed to elucidate the potential therapeutic effect of dietary nitrate.
On the other hand, it should also be recognised that other studies have not found a significant effect of dietary nitrate on blood pressure in healthy subjects( Reference Miller, Marsh and Dove 78 – Reference Kim, Moore and Maurer 81 ) or patients with cardiovascular risk factors( Reference Zand, Lanza and Garg 82 ), hypertension( Reference Bondonno, Liu and Croft 83 , Reference Bondonno, Liu and Croft 84 ), type 2 diabetes( Reference Gilchrist, Winyard and Aizawa 85 ), obesity( Reference Lara, Ogbonmwana and Oggioni 86 ) and chronic obstructive pulmonary disease( Reference Shepherd, Wilkerson and Dobson 87 ) (Table 4). This lack of a significant effect of dietary nitrate supplementation on blood pressure could be related to several factors. For example, some studies included participants taking anti-hypertensive medications and/or hypoglycaemic medications( Reference Bondonno, Liu and Croft 84 , Reference Gilchrist, Winyard and Aizawa 85 , Reference Shepherd, Wilkerson and Dobson 87 ), which could affect NO metabolism. Additionally, the recent use of antibiotics or antibacterial mouthwash was not reported in some studies( Reference Gilchrist, Winyard and Aizawa 85 , Reference Lara, Ogbonmwana and Oggioni 86 ). These are important factors that can affect the response of blood pressure to dietary nitrate and which could explain negative findings. Also, it should be noted that some patients suffering from cardiovascular disorders, obesity and type 2 diabetes may have a lower response to dietary nitrate given the impaired NO metabolism associated with these conditions. Some negative studies reported significant correlations with baseline systolic blood pressure, inferring that healthy participants with the highest baseline experienced greatest reductions( Reference Haider and Folland 79 , Reference Ashworth, Bailey and Hayward 80 ). The conclusion that can be drawn from studies reporting non-significant changes to blood pressure is that the overall understanding of the efficacy of nitrate supplementation is still unclear, especially in patients with pre-existing clinical conditions. This view is supported by a recent meta-analysis and systematic review of dietary nitrate and endothelial function, which found that increasing age, obesity and systolic blood pressure were associated with a reduced effect of nitrate supplementation( Reference Lara, Ashor and Oggioni 88 ). Further research is needed to investigate whether dietary nitrate could improve cardiovascular health in the older population with cardiovascular risk factors( Reference Lara, Ashor and Oggioni 88 ).
Table 4 Summary of research studies on nitrate and health with non-significant effects on systolic and diastolic blood pressure, 2006–2016

SBP, systolic blood pressure; DBP, diastolic blood pressure; CV, cardiovascular; COPD, chronic obstructive pulmonary disease.
* Mean nitrate dose of all studies about 600 mg/d, range 420–840 mg/d.
Amount of dietary nitrate required to promote cardiovascular health
The systematic review and meta-analysis of nitrate supplementation trials concluded that inorganic nitrate and beetroot juice supplements are associated with significant reductions in systolic blood pressure( Reference Siervo, Lara and Ogbonmwan 52 ). A range of doses was reported, either in the form of beetroot juice or nitrate salts (mean dose about 500 mg nitrate, range 157–1395 mg nitrate)( Reference Siervo, Lara and Ogbonmwan 52 ). Similar variations in methodology can be seen in studies published in the last 3 years (Table 3). The average amount of nitrate used in these trials can be calculated as about 500 mg/d (range 139–1042 mg/d). However, a word of caution must be raised in regards to some studies using beetroot juice as a supplement. A recent study by Jajja et al. ( Reference Jajja, Sutyarjoko and Lara 59 ) found that 70 ml of concentrated beetroot juice, which was expected to provide between 300 and 400 mg of nitrate, in fact yielded only 165 mg. This could be due to environmental factors (season of the year, light, irrigation of the field), the use of fertilisers and storage conditions (temperature, humidity and light), as detailed earlier. Therefore, it is important that future studies using natural supplements such as beetroot juice should directly analyse the amount of nitrate in the supplement rather than reporting values provided by the manufacturer.
Using pharmacological salts (potassium nitrate), Kapil et al. ( Reference Kapil, Milsom and Okorie 89 ) investigated the effect of two different doses providing 248 and 744 mg of nitrate, respectively. While both doses were similarly effective in reducing diastolic blood pressure, the lower dose did not show a significant effect in reducing systolic blood pressure. This suggests that a minimum amount of nitrate might be needed to induce changes in systolic blood pressure. In accord with current evidence, this threshold could be up to about 500 mg of nitrate (Table 3). This amount clearly exceeds the ADI of 3·7 mg/kg per d (about 260 mg/d for a 70 kg adult) as well as exceeding the average intake of vegetarians( Reference Lidder and Webb 27 , Reference Mitek, Anyzewska and Wawrzyniak 28 ). Furthermore, this relies on individual food choices and both omnivores and vegetarians could easily reach or exceed such levels of nitrate intake. Hord et al. ( Reference Hord, Tang and Bryan 3 ) estimated that two servings per d of vegetables such as spinach and greens provide over 1000 mg of nitrate. However, Hord’s estimates assumed a maximum nitrate content of those vegetables( Reference Hord, Tang and Bryan 3 ).
More precisely, Lidder & Webb( Reference Lidder and Webb 27 ) advocate daily consumption of at least one portion of high-nitrate vegetables to increase nitrate intake in order to lower blood pressure. Oyebode et al. ( Reference Oyebode, Gordon-Dseagu and Walker 90 ) suggest that the current UK ‘5-a-day’ message could be amended to specifically advise on numbers of portions of vegetables. This would be in line with Australian government recommendations, which advise Australians to eat five portions of vegetables and two portions of fruit daily( 91 ). For example, changing the UK message to ‘at least 5-a-day + 1 green leafy veg’ may help to remind consumers to include high-nitrate vegetables as part of their daily target.
However, it is important to note that there is a lack of human studies investigating the chronic effect of high doses of dietary nitrate on blood pressure. Previous studies have not analysed a period of nitrate supplementation beyond 1 month. Although several epidemiological studies have linked high consumption of vegetables (especially green leafy vegetables)( Reference Joshipura, Hung and Li 29 , Reference Joshipura, Hu and Manson 92 ) with a lower risk of CVD, it is not possible to state that nitrate is responsible for those effects to date. Furthermore, research using animal models has found conflicting results. Whilst a first study found that 8 weeks of nitrate (equivalent to a human dose of 350 mg/75 kg per d), reduced the mean arterial blood pressure in mice at the end of the lifespan( Reference Carlström, Persson and Larsson 48 ), a second study from the same research group did not show any effect( Reference Hezel, Liu and Schiffer 49 ). The authors suggested that long-term supplementation may have affected the nitrate homeostasis of the animals, which is supported by the lack of differences in final plasma levels of nitrite and nitrate( Reference Hezel, Liu and Schiffer 49 ). The dose used in these studies was lower than the average given in human trials, but similar to the nitrate intake reported in vegetarians. The authors also suggested that the oral microbiome could be modified under extended nitrate treatment which affected the final results( Reference Hezel, Liu and Schiffer 49 ). However, this hypothesis has not been confirmed in a recent study in human subjects, indicating that the oral microbiota of vegetarians did not differ from that of omnivores, despite significantly different dietary patterns( Reference Filippis, Vannini and Storia 93 ). In spite of the fact that these animal studies are difficult to replicate in human subjects, further research will be necessary to investigate the effects of chronic exposure to dietary nitrate and the extent to which long-term adaptations occur.
The UK Department of Health established dietary reference values (DRV) for essential nutrients to clearly define, where possible, the contexts in which intakes could be deficient, adequate or potentially excessive across different population groups( 94 ). No DRV has been established for nitrate as it is not currently considered as a nutrient with potential health benefits. The intake of excessive amounts of nitrate could be associated in specific contexts with an increased risk of negative health outcomes. However, the ADI for nitrate has been viewed as anachronistic and some authors have suggested it could be used to achieve an intake likely to derive cardiovascular benefits( Reference Lidder and Webb 27 ).
Implications both at population level and in population groups
Approximately 30 % of adults (about 15 million) in the UK have high blood pressure, usually defined as systolic blood pressure over 140 mmHg and diastolic blood pressure over 90 mmHg( 1 ). There is a strong relationship between blood pressure and mortality from CVD, so that an increase in adult systolic blood pressure by 20 mmHg is associated with doubling of death rate from CVD such as stroke( 95 ). Conversely, a reduction of 10 mmHg in systolic blood pressure is associated with about 40 % lower risk of stroke death throughout middle age( 95 ). A smaller reduction of just 5 mmHg across the population could reduce stroke mortality by 14 %( Reference Appel, Brands and Daniels 96 ). This 5 mmHg reduction across the population in England could save 45000 quality-adjusted life years over 10 years as well as £850 million spent on health and social care services( 2 ). In addition, stroke is one of the largest causes of disability and over one-third of survivors are dependent on others, such as family relatives, for their care( 97 ). This places an additional burden on carers as well as the economy, due to lost income. The total financial cost of stroke to UK society, including loss of productivity as well as cost of health care, is approximately £9 billion each year( Reference Saka, McGuire and Wolfe 98 ). Prevention of high blood pressure has been identified as a key factor to improve the nation’s health, by a combination of improvements in diet at a population level as well as encouraging individual behaviour change in diet, physical activity, alcohol and smoking( 2 ). Promoting consumption of high-nitrate vegetables could be used as a low-cost yet effective measure to improve cardiovascular health across the population( Reference Lidder and Webb 27 , Reference Hobbs, George and Lovegrove 53 , Reference Gee and Ahluwalia 99 , Reference Mills, Khatri and Maskell 100 ) and this approach could be more effective than targeting high-risk individuals( Reference Rose 101 ).
Certain groups in the population may be at risk from receiving insufficient nitrate due to their medical condition or interference with the nitrate–nitrite–NO pathway. For example, 3–7 d of antimicrobial mouthwash, which reduces the activity of nitrate-reducing commensal bacteria, has been shown to increase blood pressure( Reference Kapil, Haydar and Pearl 39 , Reference Bondonno, Liu and Croft 102 ). This fact could be important in those at risk of CVD and future studies are needed to investigate the long-term effects of using antibacterial mouthwash in different population groups.
Hypertension is both a cause and consequence of chronic kidney disease. In the USA, it has been estimated that the prevalence of renal failure among subjects with prehypertension and undiagnosed hypertension could be 17 and 22 %, respectively( Reference Crews, Planting and Miller 103 ). Renal patients who require dialysis are another potentially ‘at-risk’ group, as haemodialysis effectively removes both nitrite and nitrate from blood and saliva which in turn could reduce NO bioavailability( Reference Bryan, Torregrossa and Mian 104 ). Consequently, this could be a cause of the increased risk of cardiovascular mortality in this group of patients.
Another potential ‘at-risk’ group are patients who are on intensive care units, who are often intubated so that their ability to swallow their saliva is affected. The formation of NO has been found to be almost abolished in this situation in the stomach, and nitrite supplementation has been shown to restore NO activity in this patient group( Reference Björne, Govoni and Törnberg 105 ). However, critically ill patients often receive nutritional support as either enteral or parenteral nutrition, which contain little or no nitrate( Reference Bryan 26 , Reference Weitzberg, Hezel and Lundberg 106 ). In this scenario, with reduced saliva production as well as the effect of broad-spectrum antibiotics on oral bacteria, NO production in the stomach can be limited. This could explain the high incidence of infection in these patients( Reference Weitzberg, Hezel and Lundberg 106 ).
Finally, a recent review highlighted the protective effects of foods containing polyphenols, vitamin C and vitamin E against N-nitrosamine formation and reiterated the inverse association between dietary nitrate and stomach cancer( Reference Ahluwalia, Gladwin and Coleman 107 ). However, it recommended the need for further research into nitrate consumption in specific populations. For example, the nitrate content of drinking water (especially from private wells in rural areas of the USA) should be recorded in prospective cohort studies to determine whether individuals with a high water nitrate intake are either at increased risk of developing cancer or benefit from improved cardiovascular health( Reference Ahluwalia, Gladwin and Coleman 107 ). In addition, future cancer research should focus on population groups who may be at increased risk of N-nitrosamine formation such as smokers and those taking large quantities of nitrate or nitrite supplements which may not contain protective polyphenols and vitamins( Reference Ahluwalia, Gladwin and Coleman 107 ). This review noted that dietary nitrate from vegetables should not be included in such cancer research studies( Reference Ahluwalia, Gladwin and Coleman 107 ).
Conclusion
The ADI for nitrate of 3·7 mg/kg body weight was set several decades ago due to fears of carcinogenicity arising from animal studies as well as the incidence in methaemoglobinaemia in babies. Subsequent legislation significantly affects the food, water and agriculture industries today. However, the findings of the original animal studies have been questioned and discounted in light of more recent research. In contrast, epidemiological studies suggest that green leafy vegetables, which are naturally high in nitrate, promote cardiovascular health. Vegetarians have been reported to have high intakes of nitrate and also have reduced risks of cancer, lower blood pressure, less CVD and lower mortality rates. There is increasing evidence from human intervention studies to support the hypothesis that dietary nitrate effectively reduces blood pressure, amongst other outcomes, in both healthy subjects and those with clinical conditions. Others studies have not confirmed these findings and this could be due to reduced nitrite and NO bioavailability, especially in clinical populations, amongst other methodological reasons. However, a small, population-wide downward shift in systolic blood pressure, as suggested by a systematic review and other more recent studies, could significantly reduce the incidence of CVD such as stroke. From this viewpoint, some authors have called for nitrate to be seen as a nutrient, rather than a contaminant. The amount of nitrate needed to reduce blood pressure could be over and above the ADI, although more studies are needed and the results of longer-term trials are awaited. This level of intake could be partly achieved by eating at least one portion of high-nitrate vegetables daily, in addition to other vegetables. This would be consistent with current UK Government advice to eat more fruit and vegetables.
The implication of the evidence presented and discussed is that the current classification of nitrate as a contaminant needs to be re-examined. Due to beneficial effects on systolic blood pressure, dietary nitrate may in future be recognised as a nutrient necessary for vascular health. If dietary nitrate is indeed a ‘new’ nutrient, then dietary strategies could be designed to reduce the risk of hypertension and CVD such as stroke.
Acknowledgements
This research received no specific grant from any funding agency, commercial or non-profit sectors.
A. A. conducted the review of the literature and drafted the manuscript. R. B. contributed to the drafting of the manuscript. Both authors made a critical review of the draft.
There are no conflicts of interest.







