Combustion synthesis, or Self-propagating High- temperature Synthesis (SHS), is currently being used by the Center for Commercial Applications of Combustion in Space (CCACS) at the Colorado School of Mines to produce advanced porous materials for several important applications. These materials include ceramic, inter- metallic, and metal- matrix composites that can be used for orthopedic implants, heat exchanger and damping systems and micro-and macro-filter applications. Functionally graded materials, both in porosity and composition, can be produced using a range of combustion synthesis reactions systems. There are multiple factors that contribute to the final SHS product, e.g. reactant stoichiometry, initial relative density and pre-heat. The synthesis of nickel-titanium (NiTi) intermetallic compounds and composites is of considerable interest due to the ability to create a porous, shape memory and super-elastic alloy with high corrosion resistance. The synthesis effects of adding a carbon reactant so as to modify the reaction products and reaction exothermicity have been studied through the use of two different reaction stoichiometries involving elemental nickel, titanium and carbon. This paper outlines the synthesis of NiTi intermetallic composites based on the following SHS chemical reaction:
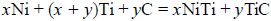
The effect of the carbon reactant and the initial sample green density on the apparent porosity, bulk density, pore size and pore distribution of the final materials has been studied and is presented within this paper. A NiTi- TiC intermetallic ceramic composite has been synthesized that is functionally graded in both composition and porosity: the latter grading being due to buoyancy and gas evolution effects. Proposed kinetic mechanisms that drive this synthesis process and control the graded structure are discussed in detail.