No CrossRef data available.
Published online by Cambridge University Press: 21 February 2011
The spreading of molten 60Sn40Pb drops on higher melting point Pb-Sn alloy substrates (3 to 10 wt.% Sn) was investigated for reflow temperatures of 205° to 300°C. Following melting the drop assumed the form of a slightly flared, spherical cap with some penetration into the substrate beneath the contact area. The effects of time and temperature on the contact angle θ and the depth of penetration h were of the form
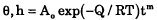
where the apparent activation energy Q was 4.2 kcal/mole for θ and 16 kcal/mole for h. The time exponent m (negative for θ and positive for h) decreased with temperature from ∼ 0.2–0.3 at 205°C to ∼0.05 at 260° and then increased again at higher temperatures. The magnitude of Q for θ is in accord with that for the viscosity of molten Pb-Sn alloys and that for h with a combined liquid-solid diffusion involved in the dissolution. Further work is however needed to identify unequivocally the mechanisms which govern the wetting in these duplex Pb-Sn alloy systems.