Article contents
Zn-enriched PtZn nanoparticle electrocatalysts synthesized by solution combustion for ethanol oxidation reaction in an alkaline medium
Published online by Cambridge University Press: 17 April 2018
Abstract
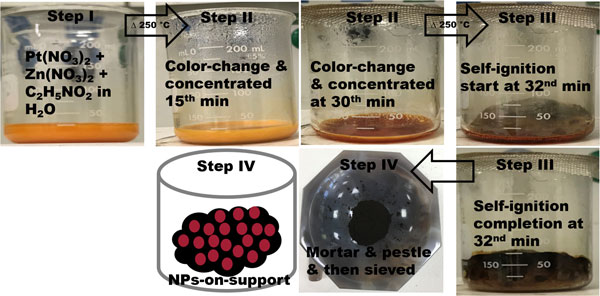
This work focuses on the syntheses of Zn-enriched PtZn nanoparticle electrocatalysts by solution combustion for ethanol oxidation reaction (EOR). Analytical techniques of x-ray diffraction, transmission electron microscopy (TEM), scanning electron microscopy, TEM/scanning TEM-energy dispersive x-ray spectroscopy, and x-ray photoelectron spectroscopy are used for the characterization of electrocatalysts. Cyclic voltammetry and chronoamperometry are applied for the electrocatalysis of C2H5OH and stability test in an alkaline medium, respectively. Electrochemical data show that PtZn/C has improved electrocatalytic activity by ~2.3 times compared with commercial Pt/C, in addition to having earlier onset potential and better stability for EOR. The variation of fuel amount in the synthesis has affected crystallite sizes, electronic, and electrochemical properties in electrocatalysts.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 9
- Cited by



