Article contents
Surface analyses of amorphous aluminum oxides with AlO6 clusters
Published online by Cambridge University Press: 10 November 2020
Abstract
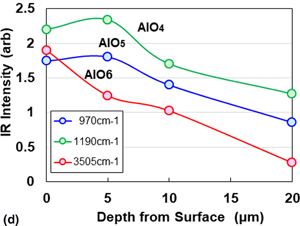
An amorphous aluminum oxide supercapacitor can store a large amount of electric storge on the uneven surfaces with AlO6 clusters. The amount of stored electricity increases with decreasing convex diameter d and depth of valley h. The nondestructive detection of AlO6 clusters on a surface with (Al0.91Y0.09)O1.66 oxide layer at a depth of 0.5 μm was determined based on a 3505 cm−1 peak band in the Fourier transform-infrared (FT-IR) spectrum and one 1047 cm−1 peak in the microRaman spectrum. The discharging time (T) could be expressed as T = 1.388 × 100.019 I. Thus, we can evaluate the amount of electricity by the nondestructive detection methods such as FT-IR and the microRaman spectra.
- Type
- Research Letters
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 3
- Cited by



