Published online by Cambridge University Press: 09 October 2017
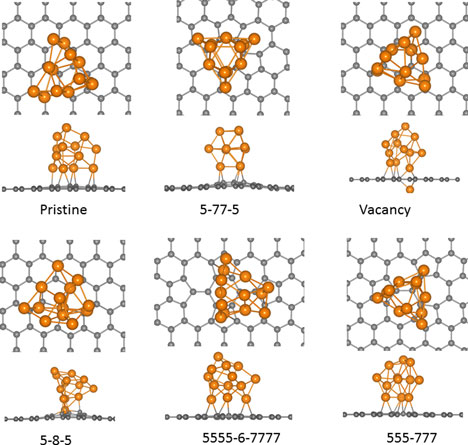
First principles simulations and global optimization predict new mode of binding of Pt clusters with defects on graphene that significantly enhances their stability. Pt clusters were found to firmly bind to monovacancies in configuration transacting the vacancy site, while retaining the integrity of the cluster. Diffusion calculations support tight anchoring of Pt cluster to monovacancy. Pt cluster adsorbed on pristine graphene or other common defects exhibit a different mode of adsorption and only decorate one side of graphene. This study reveals strong influence of defect chemistry on the structure and mobility of Pt nanoclusters adsorbed on graphene and have important implications for catalytic and gas sensing applications.