Published online by Cambridge University Press: 14 October 2011
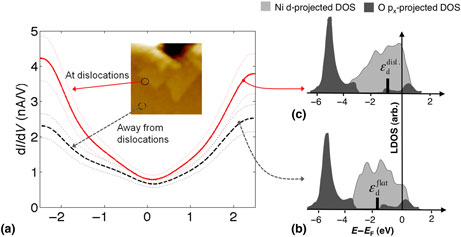
Using in situ indentation, we show that highly localized and well-defined mechanical deformation can be coupled with structural and electronic characterization in the scanning tunneling microscope. Dislocations induced in Ni(100) were topographically imaged and probed by scanning tunneling spectroscopy to assess their effect on local surface electronic structure. Compared with undamaged terraces, dislocation regions exhibited a significant increase in local density of states near the Fermi level, and enhanced reactivity toward oxidation. In the context of the d-band electronic structure model, we suggest that the undercoordination of atoms and residual strain resulting from plastic deformation serve to locally accelerate adsorption-driven chemical reactions with species such as molecular oxygen.