Introduction
Recently, photovoltaic cells with light-harvesting organo-lead halide perovskite compounds have been extensively studied owing to their tremendously improved photovoltaic conversion efficiency, light weight, high processability, and inexpensive fabrication.[Reference Kojima, Teshima, Shirai and Miyasaka1–Reference Kim, Uchida, Matsushita, Cojocaru, Jono, Kimura, Matsubara, Shirai, Ito and Matsumoto4] The cells with the perovskite compounds, for example, composed of mixed cations of methylamine, formamidine, cesium, and rubidium, have provided high photovoltaic conversion efficiency beyond 20%.[Reference Pang, Hu, Zhang, Lv, Yu, Wei, Qin, Xu, Liu and Cui5–Reference Bella, Griffini, Correa-Baena, Saracco, Grätzel, Hagfeldt, Turri and Gerbaldi7] That was ascribed to their wide light-absorption range and efficient charge-separation process, which were attributed to the formation of the high-quality perovskite layer.[Reference Cojocaru, Uchida, Sanehira, Nakazaki, Kubo and Segawa8,Reference Guo, Mccleese, Kolodziej, Samia, Zhao and Burda9] However, the durability of the perovskite compounds under light irradiation in ambient air (even dry) is very poor for their development in practical use.[Reference Asghar, Zhang, Wang and Lund10,Reference Niu, Guo and Wang11] Haque and colleagues have reported that organo-lead halide perovskites are decomposed in the presence of oxygen without any moisture under light irradiation, according to the suggested mechanism [Scheme 1(a) and 1(b)].[Reference Aristidou, Sanchez-Molina, Chotchuangchutchaval, Brown, Martinez, Rath and Haque12] The organo-lead halide perovskite, typically represented as CH3NH3PbI3, reacts with superoxide anion radical 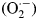 $({\rm O}_2^{\cdot -} )$, generated with an excited electron supplied from the light-irradiated perovskite layer to yield methylamine, lead iodide, and other fragments.[Reference Aristidou, Sanchez-Molina, Chotchuangchutchaval, Brown, Martinez, Rath and Haque12,Reference Wang, Mujahid, Duan, Wang and Xue13] An elimination of the superoxide anion radical could definitely improve the durability of both the perovskite layer and its cells.
$({\rm O}_2^{\cdot -} )$, generated with an excited electron supplied from the light-irradiated perovskite layer to yield methylamine, lead iodide, and other fragments.[Reference Aristidou, Sanchez-Molina, Chotchuangchutchaval, Brown, Martinez, Rath and Haque12,Reference Wang, Mujahid, Duan, Wang and Xue13] An elimination of the superoxide anion radical could definitely improve the durability of both the perovskite layer and its cells.

Scheme 1. (a) Chemical structure of phenolic compounds. (b) Degradation mechanism of the methylammonium lead iodide perovskite with superoxide anion radical. (c) Elimination mechanism of superoxide anion radical with a phenolic antioxidant.
Sterically hindered 2,6-di-tert-butylphenol derivatives are widely used as phenolic antioxidant additives for chemical commodities, such as plastics, fibers, and foods, owing to their low environmental load and negligible effects on human bodies.[Reference Dimitrios14–Reference Villamena, Das and Nash16] The phenolic antioxidants quench the highly reactive superoxide anion radical, as shown in Scheme 1(c), where one phenolic antioxidant molecule reacts with two superoxide anion radicals to prevent the oxidative degradation of organic materials.[Reference Shahidi, Janitha and Wanasundara17,Reference Lien, Ren, Bui and Wang18] We have recently reported that a small amount incorporation of antioxidizing radical polymer, poly(1-oxy-2,2,6,6-tetramethylpiperidin-4-yl methacrylate), in the perovskite layer on a perovskite-based solar cell was very effective for enhancing the durability of the cell without any reduction of the photovoltaic performance.[Reference Suwa, Oyaizu, Segawa and Nishide19] In addition, we have discussed the mechanism to prevent the degradation of the perovskite layer with ambient oxygen.[Reference Suwa, Oyaizu, Segawa and Nishide19] Furthermore, recently, Park et al. reported the facile incorporation of 2,6-di-tert-butyl-4-cresol into perovskite layers to form high-quality and highly durable perovskite crystal layers.[Reference Kumar, Choi, Kang, Oh, Lee, Seo, Jeong, Kwon, Seok, Yang and Park20] Pursuing more effective antioxidizing 2,6-di-tert-butylphenol derivatives for the perovskites and studying their antioxidizing mechanism could lead to the further improvement in the durability of photovoltaic cells.
In this paper, we designed stabilizing additives for perovskite compounds used for solar cells; moreover, we carefully selected two phenolic compounds among the commercially available antioxidants and incorporated them in mixed-cation perovskite layers to improve their durability against oxygen. A small amount incorporation of antioxidants did not decrease the photovoltaic performance of the solar cells, and the role of the antioxidants in suppressing the decomposition of the perovskite layers was further discussed. The facile incorporation of the antioxidants into the perovskite layers could provide an effective method to guarantee the durability of the organo-lead halide perovskite compounds in their solar cells.
Materials and methods
2,6-Di-tert-butyl-4-cresol (dibuthylhydroxytoluene, BHT), pentaerythritol tetrakis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate] (PTP), and all perovskite precursors, described below, were purchased from Tokyo Chemical Industry Co.
Perovskite solar cells were fabricated using a previously reported procedure.[Reference Suwa, Tanaka, Oyaizu and Nishide21–Reference Okada, Suga, Oyaizu, Segawa and Nishide23] A compact TiO2 layer was formed on an edged fluorine-doped tin oxide (FTO) conductive substrate (Atock Co.) by spray-coating an ethanol solution of titanium diisopropoxide bis(acetylacetonate) (Sigma-Aldrich Co.) on it. A mesoporous TiO2 (m-TiO2) underlayer was formed using an ethanol solution of Ti paste (PST-18NR, JGC Catalysts and Chemicals Ltd.) via spin-coating it on the compact TiO2/FTO substrate at 4000 rpm for 20 s followed by annealing at 100 °C for 10 min and at 500 °C for 1 h.
A mixed-cation perovskite precursor solution for the photovoltaic devices was prepared by dissolving formamidine hydroiodide (172 mg), methylamine hydrobromide (22.4 mg), PbI2 (507 mg), PbBr2 (80.7 mg), CsI (16.3 mg), and RbI (13.4 mg) powders in a mixture of N,N′-dimethylformamide (DMF) (800 µL), dimethyl sulfoxide (DMSO) (114 µL), and acetonitrile (86 µL), as previously reported.[Reference Okada, Suga, Oyaizu, Segawa and Nishide23] Then, the 0.1 wt% phenolic antioxidant (versus the perovskite) was added to the perovskite precursor solutions. The mixture was stirred for 1 h and subsequently spin-coated on the m-TiO2 underlayer at 1000 rpm for 10 s and at 6000 rpm for 20 s to form the antioxidant-incorporated perovskite layer. The perovskite surfaces were not passivated by alkylammonium halides, as previously reported,[Reference Okada, Suga, Oyaizu, Segawa and Nishide23] for confirming the accurate durability of the cells.
2,2,7,7-Tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene (spiro-OMeTAD) (Sigma-Aldrich Co., 85.8 mg in 1 mL of chlorobenzene) with 19.4 µL of lithium bis(trifluoromethylsulfonyl) imide (Tokyo Chemical Industry Co., 520 mg/mL in acetonitrile), 33.8 µL of tert-butylpyridine, and 31.6 µL of tris(2-(1H-pyrazol-1-yl)-4-tert-butylpyridine)cobalt(III) tri[bis(trifluoromethane)sulfonimide] (Tokyo Chemical Industry Co., 100 mg/mL in acetonitrile) was spin-coated at 4000 rpm for 20 s on the perovskite layer as the hole-transporting layer. Finally, the electrode, which consisted of the 100 nm Au layer, was deposited on the hole-transporting polymer layer using a vacuum coater (VPC-1100, ULVAC, Inc.). The fabricated perovskite cells were kept in a dry room for 1 day before their performance measurements.
Current–voltage (J–V) measurements under irradiation were conducted using a solar cell evaluation system (YQ-2000, JASCO Co.) and a solar simulator (CEP-2000MLQ, Bunkoukeiki Co.). All J–V curves in this study were obtained at the scan rate of 200 mV/s under the light intensity of AM 1.5 standard solar emission (1 SUN irradiation) with an active area of 0.03 cm2. The durability of the cells after sequential light irradiation was measured by checking J–V characteristics at different times.
The formation of superoxide anion radical on the surface of the perovskite layers (surface area of 3.75 cm2) was monitored by coating the surface with the 0.317 µM toluene solution of hydroethidine (Kanto Chemical Co.), which is a fluorescent probe that selectively reacts with superoxide anion radical to yield 2-hydroethidium and presents strong fluorescence at the excitation and emission wavelengths of 520 and 610−640 nm, respectively.[Reference Aristidou, Sanchez-Molina, Chotchuangchutchaval, Brown, Martinez, Rath and Haque12,Reference Peshavariya, Dusting and Selemidis24,Reference Benov, Sztejnberg and Fridovich25] The fluorescence intensity of 2-hydroethidium after 90 min of 1 SUN irradiation and oxygen exposure was measured using a fluorescence spectrophotometer (F-7000, Hitachi, Ltd.).
An x-ray diffractometer (XRD, LINT-UltimaIII, Rigaku Co.) using the focused beam method spectroscopy was utilized to perform the x-ray spectroscopy analysis. A scanning electron microscope (SEM, SU8000, Hitachi, Ltd.) was used to obtain surface and cross-sectional images of the perovskite layers and their solar cells. A contact angle analyzer (DSA-25S, KRÜSS GmbH) was employed to measure the water contact angles upon the perovskite layers.
Results and discussion
Small amounts of phenolic antioxidants (0.1 wt% versus the perovskite) were incorporated into the mixed-cation lead halide perovskite layers with the thickness of approximately 500 nm upon the m-TiO2 underlayers via simple spin-coating of their DMF/DMSO precursor solution (see the Materials and methods section). The small amounts of the antioxidants did not impede the formation of the perovskite layers and facilitated the generation of dense perovskite grains, as observed in the cross-sectional and surface SEM images (Supplementary Fig. S1). The average sizes of the perovskite crystallites obtained in the absence of additives and after the incorporation of BHT and PTP into the perovskite layer were 61, 61, and 57 nm, respectively (Supplementary Fig. S2, black). These values were estimated from the full width at half maximum of their XRD patterns using Scherrer's equation (D = κλ/Bcosθ, where D is the diameter of the grain, κis Scherrer's constant, λis the wavelength of x-ray, B is the full width at half maximum, and θ is the Bragg angle, respectively)[Reference Suwa, Oyaizu, Segawa and Nishide19] and were not affected by the incorporation of the phenolic additives. The XRD patterns of the perovskite layers after 12 h of 1 SUN irradiation and oxygen exposure are also shown in Supplementary Fig. S2 (red). The XRD patterns revealed that the formation of PbI2, which is one of the decomposed fragments of the perovskite compound, was greatly suppressed by the incorporation of the antioxidants, whereas the decomposition of the perovskite layer that did not contain additives resulted in the formation of a significant amount of PbI2.
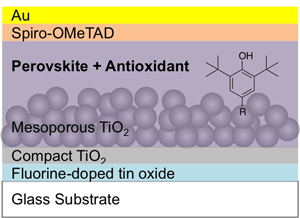
Solar cells were fabricated with a perovskite layer using the so-called anti-solvent method and the 4-cation perovskite system composed of methylamine, formamidine, cesium, and rubidium (see the Materials and methods section). The perovskite layer was deposited on the m-TiO2 underlayer that was formed on the FTO substrate covered with a compact TiO2 layer. Then, a hole-transporting layer of spiro-OMeTAD and the Au electrode were spin-coated and deposited, respectively, on the perovskite layer (Fig. 1). The photovoltaic performance of the cells with and without the antioxidants is presented in Fig. 2(a) and Table I, respectively. The conversion efficiency of the cells was not significantly reduced due to the incorporation of the small amounts of the antioxidants, and they also showed the high reproducibility [inset of Fig. 2(a)].

Figure 1. Configuration of the perovskite solar cell fabricated with the organo-lead halide perovskite layer with a small amount incorporation of the phenolic antioxidant.

Figure 2. (a) J–V curves of the solar cells fabricated with the perovskite in the presence of 0.1 wt% phenolic antioxidants (◯: w/o, ●: BHT, and □: PTP). The measurements were executed as reverse scans after 1 day from the cell fabrication. Inset: the photovoltaic conversion efficiency of 12 cells. (b) Time course of the efficiency of the unsealed perovskite solar cells (average of eight cells) under 1 SUN light irradiation and dry air exposure.
Table I. Top photovoltaic conversion efficiency (η) of the perovskite solar cells fabricated with the phenolic antioxidants.
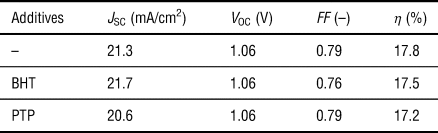
J SC: short-circuit current density; V OC: open circuit voltage; FF: fill factor.
The time course of the conversion efficiency of the cells fabricated with the antioxidant-incorporated perovskite layers under consecutive 1 SUN irradiation under dry air is shown in Fig. 2(b). The incorporation of phenolic antioxidants in the perovskite layers clearly enhanced the durability of the obtained cells. The elimination of superoxide anion radical at the surface of the antioxidant-incorporated perovskite layers was studied by using a fluorescent probe that reacts with superoxide anion radical, as described in one of our previously published papers.[Reference Suwa, Oyaizu, Segawa and Nishide19] The amount of the superoxide anion radical formed by the PTP-incorporated perovskite layer was more than five times lower than that formed at the surface of the antioxidant-free perovskite layer (Supplementary Fig. S3); therefore, the incorporated antioxidant suppressed the decomposition of the perovskite layer.
The incorporation of hydrophobic phenolic antioxidants into the perovskite layers also improved their water-repelling properties. The water contact angles of the perovskite layers increased from 54° for the antioxidant-free perovskite layer to 71° and 82° for the BHT- and PTP-incorporated perovskite layers, respectively (Supplementary Fig. S4). The more hydrophobic structure of PTP compared to that of BHT could have contributed to the higher water contact angle of the PTP-incorporated perovskite layer compared to that of the BHT-incorporated perovskite layer. The water repellency of the perovskite layers could contribute to increasing the durability of the perovskite-based cells under humid conditions.
In conclusion, the incorporation of phenolic antioxidants, namely BHT and PTP, into the perovskite layers could suppress the degradation of the perovskite compounds and enhance the durability of perovskite-based cells by capturing superoxide anion radical generated at the surface of the perovskite layers. In particular, PTP effectively enhanced both the antioxidizing and water-repelling properties of the perovskite layer analyzed in this study and further improved the long-term durability of the cells in humid air, including oxygen and water. The incorporation of these phenolic compounds into perovskites to improve the cell durability was very versatile approach without complex processes and was not compromising the photovoltaic efficiency of the cells.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1557/mrc.2020.25.
Acknowledgments
This research was partially supported by “Research and Development of Innovative New Structure Solar Cells” from NEDO, Japan. K.S. acknowledges the Leading Graduate Program in Science and Engineering at Waseda University from MEXT, Japan.