No CrossRef data available.
Published online by Cambridge University Press: 30 August 2016
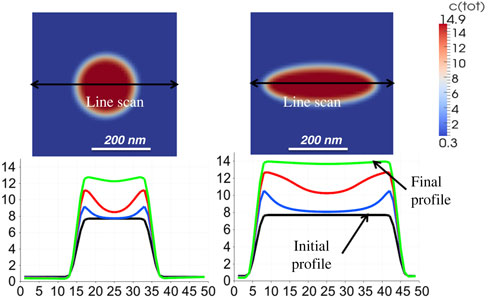
When two materials interact, the processes between the phases determine the functional properties of the compound. Pivotal interface phenomena are diffusion and redistribution of atoms (molecules). This is especially of interest in Lithium ion batteries where the interfacial kinetics determines the battery performance and impact cycling stability. A new phase field model, which links the atomistic processes at the interface to the mesoscale transport by a redistribution flux controlled by the so called ‘interface permeability’ was developed. The model was validated with experimental data from diffusion couples. Calculations of the concentration profiles of the species at the electrode–electrolyte interface are reported. Active particle size, morphology and spatial arrangement were put in correlation with diffusion behavior for use in reverse engineering.