Article contents
PCBM nanoparticles as visible-light-driven photocatalysts for photocatalytic decomposition of organic dyes
Published online by Cambridge University Press: 27 December 2018
Abstract
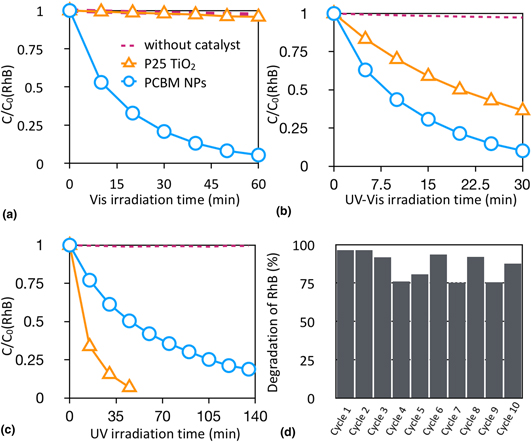
[6,6]-Phenyl-C61-butyric acid methyl esters (PCBM) have emerged in recent years as important building blocks for photovoltaic devices. However, the potential of PCBM itself as a photocatalyst has not been reviewed. Here, we demonstrate PCBM nanoparticles (NPs) fabricated by the reprecipitation method as suitable photocatalysts for an effective visible-light-driven photocatalytic degradation for organic dyes. An enhanced catalytic performance of PCBM can be achieved by a simple annealing process. The present PCBM NPs outperform the state-of-the-art P25 TiO2 and therefore highlights its potential as promising small molecule organic semiconductor photocatalysts with high photocatalytic activity and good long-term stability.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
Current address: Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK.
References
- 6
- Cited by


