Published online by Cambridge University Press: 13 July 2018
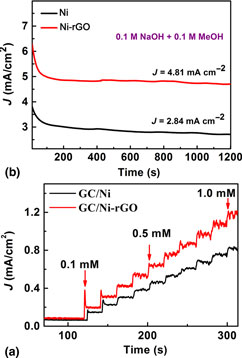
Metal–graphene composites are sought after for various applications. A hybrid light-weight foam of nickel (Ni) and reduced graphene oxide (rGO), called Ni-rGO, is reported here for small molecule oxidations and thereby their sensing. Methanol oxidation and non-enzymatic glucose sensing are attempted with the Ni-rGO foam via electrocatalytically, and an enhanced methanol oxidation current density of 4.81 mA/cm2 is achieved, which is ~1.7 times higher than that of bare Ni foam. In glucose oxidation, the Ni-rGO electrode shows a better sensitivity over bare Ni foam electrode where it could detect glucose linearly over a concentration range of 10 µM to 4.5 mM with a very low detection limit of 3.6 µM. This work demonstrates the synergistic effects of metal and graphene in oxidative processes, and also shows the feasibility of scalable metal–graphene composite inks development for small molecule printable sensors and fuel cell catalysts.
These authors contributed equally to this work.