Published online by Cambridge University Press: 31 March 2020
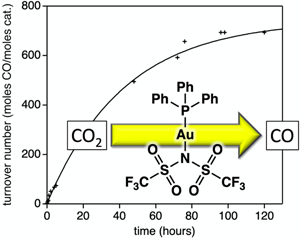
Five Au complexes are evaluated for the reduction reaction of CO2 via cyclic voltammetry and in a photocatalytic system. Electrochemically, the complexes were all evaluated for pre-association with CO2 prior to electrochemical reduction and for thermodynamic favorability for CO2 reduction in photocatalytic systems. The complexes were evaluated in photocatalytic reactions using an Ir-based photosensitizer and a sacrificial electron donor for the conversion of CO2 to CO. Au-complex counterion effects on the photocatalytic reaction were analyzed by varying weakly coordinating counterions with significant performance changes noted. At low Au-complex concentrations, a high TON value of 700 was observed.